Abstract
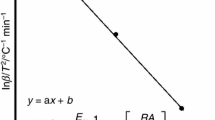
A comparative evaluation of different biomasses allows the choice that presents the best potential as fuel for energy production. The knowledge of the thermal and kinetics parameters of the biomass in the process of thermal conversion is fundamental as their chemical and physical characterization. Various methodologies have been developed for the determination of kinetic parameters as apparent activation energy and reaction order from the thermogravimetric analysis. In this work, the apparent activation energy needed to break the bonds of hemicelluloses and cellulose of rice husk and elephant grass during the thermal conversion was evaluated according to the kinetics models of Flynn and Wall and Model Free Kinetics developed by Vyazovkin. The biomass elephant grass and rice husk were characterized for moisture, ash and volatile matter by ASTM E871, ASTM E1755, ASTM E872, respectively, and fixed carbon by difference. The percentage of carbon, hydrogen, nitrogen, and oxygen were determined by ultimate analysis. The elephant grass showed to be more suitable for production of bio-oil through pyrolysis due to the higher percentage of volatile, less ash content and less energy required to break the bonds of hemicellulose and cellulose than rice husk in the thermal conversion process.




Similar content being viewed by others
References
Ilmam T, Capareda S. Characterization of bio-oil, syn-gas and bio-char from switchgrass pyrolysis at various temperatures. J Anal Appl Pyrol. 2012;93:170–7.
Wang S, Jiang XM, Wang Q, Ji HS, Wu LF, Wang JF, Xu SN. Research of specific heat capacities of three large seaweed biomass. J Therm Anal Calorim. 2013. doi:10.1007/s10973-013-3141-0.
López R, Fernández C, Gómez X, Martínez O, Sánchez ME. Thermogravimetric analysis of lignocellulosic and microalgae biomasses and their blends during combustion. J Therm Anal Calorim. 2013. doi:10.1007/s10973-012-2843-z.
Omar S, Cortez LAB, Gómez EO. Estudo cinético da biomassa a partir de resultados termogravimétricos. In: An. 3. Enc. Energ. Meio Rural 2003. http://www.proceedings.scielo.br/scielo.php?pid=MSC0000000022000000200022&script=sci_arttext. Accessed 04 Sept 2013.
Cortez LAB, Lora EES, Gómez EO. Biomassa para energia. São Paulo: Ed. da Unicamp; 2008.
Mothé CG, Miranda IC. Study of kinetic parameters of thermal decomposition of bagasse and sugarcane straw using Friedman and Ozawa–Flynn–Wall isoconversional methods. J Therm Anal Calorim. 2013. doi:10.1007/s10973-013-3163-7.
Koga N. Ozawa’s kinetic method for analyzing thermoanalytical curves. J Therm Anal Calorim. 2013. doi:10.1007/s10973-012-2882-5.
Macedo CP, Negrão CAB, Macedo LGM, Zamian JR, Rocha Filho GN, Costa CEF. Kinetic study of template removal of Al-MCM-41 synthesized at room temperature. J Therm Anal Calorim. 2013. doi:10.1007/s10973-013-3267-0.
González JF, Encinar JM, Canito JL, Sábio E, Chacón M. Pyrolysis of cherry stones: energy use of the different fractions and kinetic study. J Anal Appl Pyrolysis. 2003;57:65–190.
Mui ELK, Cheung WH, Lee VKC, McKay G. Kinetic study on bamboo pyrolysis. Ind Eng Chem Res. 2008;47:5710–22.
Zhao H, Yan HX, Dong SS, Zhang Y, Sun BB, Zhang CW, Ai YX, Chen BQ, Liu Q, Sui TT, Qin S. Thermogravimetry study of the pyrolytic characteristics and kinetics of macro-algae Macrocystis pyrifera residue. J Therm Anal Calorim. 2013;111:1685–90.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Braga RM, Barros JMF, Melo DMA, Melo MAF, Aquino FM, Freitas JCO, Santiago RC. Kinetic study of template removal of MCM-41 derived from rice husk ash. J Therm Anal Calorim. 2013;111:1013–8.
Zain MFM, Islam MN, Jamil M. Production of rice husk ash for use in concrete as a supplementary cementitious material. Constr Build Mater. 2011;25:798–805.
He J, Jie Y, Zhang J, Zhang G. Synthesis and characterization of red mud and rice husk ash-based geopolymer composites. Cem Concr Compos. 2013;37:108–18.
Guozhan J, Nowakowski DJ, Bridgwater AV. A systematic study of the kinetics of lignin pyrolysis. Thermochim Acta. 2010;498:61–6.
Doyle CD. Kinetic analysis of thermogravimetric data. J Appl Polym Sci. 1962;5:285–92.
Aquino FM, Melo D MA, Santiago RC, Melo MAF, Martinelli AE, Freitas JCO, Araú jo LCB. Thermal decomposition kinetics of PrMO3 (M = Ni or Co) ceramic materials via thermogravimetry. J Therm Anal Calorim. 2011;104:701–5.
Vyazovkin S. A unified approach to kinetic processing of nonisothermal data. Int J Chem Kinet. 1996;28:95–101.
Vyazovkin S, Wight CA. Model-free and model-fitting approaches to kinetic analysis of isothermal and nonisothermal data. Thermochim Acta. 1999;340–341:53–68.
Souza MJB, Araujo AS, Pedrosa AMG, Lima SH, Fernande VJ Jr. Kinetic parameters of surfactant remotion occluded in the pores of the AlMCM-41 nanostructured materials. Thermochim Acta. 2006;443:183–8.
French R, Czernik S. Catalytic pyrolysis of biomass for biofuels production. Fuel Process Technol. 2010;91:25–32.
Shi L, Yu S, Wang FC, Wang J. Pyrolytic characteristics of rice straw and its constituents catalyzed by internal alkali and alkali earth metal. Fuel. 2012;96:586–94.
Teixeira P, Lopes H, Gulyurtlu I, Lapa N. Use of chemical fractionation to understand partitioning of biomass ash constituents during co-firing in fluidized bed combustion. Fuel. 2012;101:215–27.
Sarenbo S. Wood ash dilemma-reduced quality due poor combustion performance. Biomass Bioenergy. 2009;9:1212–20.
McKendry P. Energy production from biomass (part 1): overview of biomass. Bioresour Technol. 2002;83:37–46.
Mayer Z, Apfelbacher A, Hornung A. Effect of sample preparation on the thermal degradation of metal-added biomass. J Anal Appl Pyrol. 2012;94:170–6.
Sait HH, Hussain A, Salema AA, An FN. Pyrolysis and combustion kinetics of date palm biomass using thermogravimetric analysis. Bioresour Technol. 2012;118:382–9.
Acknowledgements
Authors gratefully acknowledge CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnologico) for financial support, LabTam – NUPPRAR for the TG analysis and Central Analítica – NUPPRAR for the Ultimate Analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Braga, R.M., Melo, D.M.A., Aquino, F.M. et al. Characterization and comparative study of pyrolysis kinetics of the rice husk and the elephant grass. J Therm Anal Calorim 115, 1915–1920 (2014). https://doi.org/10.1007/s10973-013-3503-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3503-7