Abstract
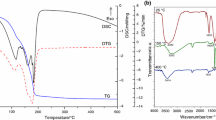
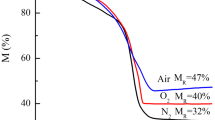
Magnetic iron oxides were prepared by precipitation of Fe(II) hydroxide using different precipitation agents: ammonia, benzylamine and sodium hydroxide, followed by oxidation with the oxygen dissolved in water. Thermal analysis, coupled with FTIR spectroscopy, has evidenced the formation of a mixture of magnetite and maghemite, with a higher content of magnetite in case of the powder synthesized with benzylamine. The stability of magnetite at oxidation by air during storage at room temperature and 60 °C was investigated by means of TG/DSC simultaneous thermal analysis, FTIR spectroscopy and X-ray diffractometry. Thermal analysis evidenced an exothermic process with mass gain in temperature range 100–190 °C, corresponding to magnetite oxidation process, but due to the superposition of other processes it could not offer quantitative information. FTIR spectroscopy has provided, especially through the first and second derivatives of FTIR spectra, the most valuable information regarding the evolution of magnetite to maghemite, due to their different characteristic bands. XRD technique has evidenced a slight shift of the main diffraction peaks at higher 2-theta values during the evolution of magnetite to maghemite. According to thermal analysis data, the powder synthesized with ammonia was completely oxidized after 15 days, while the other two powders, synthesized with benzylamine and sodium hydroxide, were completely oxidized after 110 days of keeping in air at room temperature. For a temperature of 60 °C, the oxidation was much faster; the oxidation process of the powder synthesized with benzylamine disappeared from TG/DSC curves after 1 day. All final powders were formed from nanoparticles with diameters up to 25 nm, with magnetic properties characteristic to nanometric maghemite.














Similar content being viewed by others
References
Wahajuddin M, Arora S. Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers. Int J Nanomedicine. 2012;7:3445–71.
Liu XM, Kim JK. Solvothermal synthesis and magnetic properties of magnetite nanoplatelets. Mater Lett. 2009;63:428–30.
Li YS, Church JS, Woodhead AL, Moussa F. Preparation and characterization of silica coated iron oxide magnetic nano-particles. Spectrochim Acta A. 2010;76:484–9.
Shariful-Islam MD, Kusumoto Y, Kurawaki J, Abdulla-al-Mamun MD, Manaka H. A comparative study on heat dissipation, morphological and magnetic properties of hyperthermia suitable nanoparticles prepared by co-precipitation and hydrothermal methods. Bull Mater Sci. 2012;35(7):1047–53.
Jing-San Xu, Zhu Ying-Jie. γ-Fe2O3 and Fe3O4 magnetic hierarchically nanostructured hollow microspheres: preparation, formation mechanism, magnetic property, and application in water treatment. J Colloid Interf Sci. 2012;385:58–65.
Stoia M, Păcurariu C, Istratie R, Nižňansky D. Solvothermal synthesis of magnetic FexOy/C nanocomposites used as adsorbents for the removal of methylene blue from wastewater. J Therm Anal Calorim. 2015;121:989–1001.
Shen L, Qiao Y, Guon Y, Meng S, Yang G, Wu M, Zhao J. Facile co-precipitation synthesis of shape-controlled magnetite nanoparticles. Ceram Int. 2014;40:1519–24.
Correa JR, Canetti D, Castillo R, Llopiz JC, Dufou J. Influence of the precipitation pH of magnetite in the oxidation process to maghemite. Mater Res Bull. 2006;41:703–13.
Strobel R, Pratsinis SE. Direct synthesis of maghemite, magnetite and wustite nanoparticles by flame spray pyrolysis. Adv Powder Technol. 2009;20:190–4.
Lemine OM, Omri K, Zhang B, El Mira L, Sajieddine M, Alyamani A, Bououdina M. Sol–gel synthesis of 8 nm magnetite (Fe3O4) nanoparticles and their magnetic properties. Superlattice Microst. 2012;52:793–9.
Chin SF, Pang SC, Tan CH. Green synthesis of magnetite nanoparticles (via Thermal Decomposition Method) with controllable size and shape. J Mater Environ Sci. 2011;2(3):299–302.
Ianoş R, Tăculescu A, Păcurariu C, Lazău I. Solution Combustion Synthesis and Characterization of Magnetite, Fe3O4, Nanopowders. J Am Ceram Soc. 2012;95(7):2236–40.
Hawa CY, Mohamed F, Chia CH, Radiman S, Zakaria S, Huang NM, Lim HN. Hydrothermal synthesis of magnetite nanoparticles as MRI contrast agents. Ceram Int. 2010;36:1417–22.
Wang X, Yu J, Shi G, Xu G, Zhang Z. Solvothermal synthesis of magnetite hollow submicrospheres and mesoporous nanoparticles. J Mater Sci. 2014;49:6029–38.
Can MM, Ozcan S, Ceylan A, Firat T. Effect of milling time on the synthesis of magnetite nanoparticles by wet milling. Mat Sci Eng B. 2010;172:72–5.
Maity D, Agrawal DC. Synthesis of iron oxide nanoparticles under oxidizing environment and their stabilization in aqueous and non-aqueous media. J Magn Magn Mater. 2007;308:46–55.
Sanders JP, Gallagher PK. Kinetics of the oxidation of magnetite using simultaneous TG/DSC. J Therm Anal Calorim. 2003;72:777–89.
Fajaroh F, Setyawan H, Nur A, Lenggoro IW. Thermal stability of silica-coated magnetite nanoparticles prepared by an electrochemical method. Adv Powder Technol. 2013;24:507–11.
Lepp H. Stages in the oxidation of magnetite. Am Mineral. 1957;42:679–81.
Szostko BK, Wykowska U, Satula D, Nordblad P. Thermal treatment of magnetite nanoparticle. Beilstein J Nanotechnol. 2015;6:1385–96.
Sanders JP, Gallagher PK. Thermomagnetic evidence of γ-Fe2O3 as an intermediate in the oxidation of magnetite. Thermoch Acta. 2003;406:241–3.
Haneda K, Morrish AH. Magnetite to maghemite transformation in ultrafine particles. Colloque. 1977;38:C1321–3. doi:10.1051/jphyscol:1977166.
Li YS, Church JS, Woodhead AL. Infrared and Raman spectroscopic studies on iron oxide magnetic nano-particles and their surface modifications. J Magn Magn Mater. 2012;324:1543–50.
Kim W, Suh CY, Cho SW, Roh KM, Kwon H, Song K, Shon IJ. A new method for the identification and quantification of magnetite–maghemite mixture using conventional X-ray diffraction technique. Talanta. 2012;94:348–52.
Mahadevan S, Gnanaprakash G, Philip J, Rao BPC, Jayakumar T. X-ray diffraction-based characterization of magnetite nanoparticles in presence of goethite and correlation with magnetic properties. Physica E. 2007;39:20–5.
Gorski CA, Scherer MM. Determination of nanoparticulate magnetite stoichiometry by Mössbauer spectroscopy, acidic dissolution, and powder X-ray diffraction: a critical review. Am Mineral. 2010;95:1017–26.
Gotic M, Košcec G, Music S. Study of the reduction and reoxidation of substoichiometric magnetite. J Mol Struct. 2009;924–926:347–54.
Namduri H, Nasrazadani S. Quantitative analysis of iron oxides using Fourier transform infrared spectrophotometry. Corros Sci. 2008;50:2493–7.
Daou TJ, Pourroy G, Colin SB, Greneche JM, Ulhaq-Bouillet C, Legare P, Bernhardt P, Leuvrey C, Rogez G. Hydrothermal synthesis of monodisperse magnetite nanoparticles. Chem Mater. 2006;18:4399–404.
Ueda M, Shimada S, Inaga M. Synthesis of crystalline ferrites below 60 C. J Eur Ceram Soc. 1996;16:685–6.
Legodi MA, de Waal D. The preparation of magnetite, goethite, hematite and maghemite of pigment quality from mill scale iron waste. Dyes Pigments. 2007;74:161–8.
Mihalca I, Ercuta A. Structural relaxation in Fe70Cr10.5P11.5Mn1.5C6.5 amorphous alloy. J Optoelectron Adv Mat. 2003;5:245–50.
***The Powder Diffraction File (PDF 4+) JCPDS—Joint Committee on Powder Diffraction Standards, ICC—International Center for Diffraction Data, 2012.
Olowe AA, Génin JMR. The mechanism of oxidation of ferrous hydroxide in sulphated aqueous media: importance of the initial ratio of the reactants. Corros Sci. 1991;32:965–84.
Refait Ph, Génin JMR. The oxidation of ferrous hydroxide in chloride-containing aqueous media and Pourbaix diagrams of green rust one. Corros Sci. 1993;34:797–819.
Iida H, Kosuke T, Takuya N, Tetsuya O. Synthesis of Fe3O4 nanoparticles with various sizes and magnetic properties by controlled hydrolysis. J Colloid Interface Sci. 2007;314:274–80.
Morgan B, Lahav O. The effect of pH on the kinetics of spontaneous Fe(II) oxidation by O2 in aqueous solution—basic principles and a simple heuristic description. Chemosphere. 2007;68:2080–4.
Ye X, Lin D, Jiao Z, Zhang L. The thermal stability of nanocrystalline maghemite Fe2O3. J Phys D Appl Phys. 1998;31:2739–44.
Lauer HV Jr, Ming DW, Golden DC. Thermal analysis of acicular shaped magnetite. Lunar Planetary Sci. XXXIV. 2003; http://www.lpi.usra.edu/meetings/lpsc2003/pdf/1341.pdf. Accessed 29 Sept 2015.
Fan H, Song B, Li Q. Thermal behavior of goethite during transformation to hematite. Mater Chem Phys. 2006;98:148–53.
Ishii M, Nakahira M. Infrared absorption spectra and cation distribution in (Mn, Fe)3O4. Solid State Commun. 1972;11:209–12.
Ercuta A, Chirita M. Highly crystalline porous magnetite and vacancy-ordered maghemite microcrystals of rhombohedral habit. J Cryst Growth. 2013;380:182–6.
Cambier P. Infrared study of goethites of varying crystallinity and particle size: i. interpretation of oh and lattice vibration frequencies. Clay Miner. 1986;21:191–200.
de Aragão BJG, Messaddeq Y. Peak separation by derivative spectroscopy applied to FT-IR analysis of hydrolized silica. J Braz Chem Soc. 2008;19(8):1582–94.
Stancik AL, Brauns EB. A simple asymmetric lineshape for fitting infrared absorption spectra. Vib Spectrosc. 2008;47:66–9.
Acknowledgements
This work was supported by a grant of the Romanian National Authority for Scientific Research and Innovation, CNCS–UEFISCDI, Project Number PN-II-RU-TE-2014-4-0514.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stoia, M., Istratie, R. & Păcurariu, C. Investigation of magnetite nanoparticles stability in air by thermal analysis and FTIR spectroscopy. J Therm Anal Calorim 125, 1185–1198 (2016). https://doi.org/10.1007/s10973-016-5393-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5393-y