Abstract
The wood biomass obtained from four tree species: spruce, beech, willow and alder was studied. Sawdust obtained by sawing of wood was dried in a convective dryer at 80 °C, without drying-agent flow. The measurements taken with oven-drying method covered determination of initial moisture and its detailed changes during entire drying process. Both DSC curves and spectral data from infrared spectra registered in the classic MIR range were also used to monitor change in water content in studied samples within entire drying process. Results obtained suggest that the DSC curves can be efficiently used as a discriminant to distinguish dry and wet biomass samples, as significant differences within courses of specific DSC curves of woody biomass before and after drying process occurred. Calorimetrically measured values of enthalpy of evaporation of water and water content in studied biomass samples were directly proportional to high determination coefficient, i.e. 0.9653. Statistical analysis proved quantitative directly proportional correlation between content of water measured with oven-drying method and IR spectral data of studied samples.
Similar content being viewed by others
Introduction
Due to undesired environmental effects related to fossil fuels exploitation, the contribution of renewable energy sources will continue its increase in the near-future energy scheme [1]. The European Community undertook an ambitious programme aimed at improving the sustainability of energy use across Europe. The European Union and its Member States encourage use of renewable sources of energy as well as improvement of use energy efficiency [2]. Modern industrialized societies function within a series of trade and political agreements with a variety of countries, trading blocs, and economical and political alliances. Bioenergy use, with its associated activities, production, harvesting and utilization, is integrated with political, economical and environmental agreements. Recently, many countries have developed enhanced environmental agreements, conventions and protocols designed to address issues of sustainability of resources and communities [3]. The European Commission also released a proposal for a directive on the promotion of the use of renewable energy. It addresses all sectors of the renewable energy industries, helping them to reach the Commission’s target of 20 % of Europe’s energy produced by renewable sources by 2020 [2]. Unlike wind, solar and hydroenergy power plants utilizing renewable energy source for power generation, power plants using biomass, biogas and geothermal fuels generate thermal energy as a by-product [4].
Biomass is the term used to describe all biologically produced matter [5]. Wood and energy crops, as well as agricultural and forest residues, which are the main renewable energy sources, are typical examples of biomass [6, 7]. Biomass, mainly in the form of wood, is the oldest form of energy used by humans. Traditionally, biomass has been utilized through direct combustion, and this process is still widely used in many regions of the globe [5]. Nowadays, biomass is also considered one of the most important renewable energy sources due to void carbon dioxide emission that may affect the global climate and that it involves the use of waste feedstocks and allows the increase in employment in rural areas. In the future, biomass combustion will play an important role in energy production to obtain electricity or heating [1]. Wood biomass has therefore attracted much attention recently as an environmentally friendly resource of energy since CO2 emissions and fossil fuel shortage are serious environmental issues [5, 8]. Wood fuel is considered “carbon-neutral” because while carbon dioxide is emitted when wood is combusted, the very same amount of carbon dioxide is absorbed when the tree grows [9]. The overall process sustains environment natural equilibrium. Apart from the neutral CO2 emission cycle associated with biomass use as fuel, the residues are of lower nitrogen and sulphur content than fossil fuels, and do not contribute to increase “acid rains” in the areas surrounding places of combustion [1]. Therefore, the main advantage of these fuels over the fossil ones is that there is neither SO2 nor NOx emission. One common form of biomass is wood chips, which are a by-product of the forest industry. Wood chips stored in piles before combustion contain large amounts of water on a dry matter, which demands thorough drying in order to achieve proper material for effective combustion [10]. The MC in wood has a significant influence on fuel quality because the degradation of the woody biomass is strongly influenced by the MC in the material [9]. The raw biomass usually contains 30–60 % of moisture. The material needs to be dried to circa 10–15 % MC since dry biomass provides considerable benefits for combustion, including improved operational parameters of boiler and increased energy efficiency as well as reduced flue gas emissions, compared to fuels with high moisture [11]. The drying means by definition removal of a liquid (water) from a material through thermal treatment. The main parameters controlling drying are: the temperature and pressure of the drying medium, the slip velocity and, with air as the drying medium, the humidity. The energy required for the evaporation of water can be supplied to the material in different ways: through conduction, convection, radiation and microwaves [12]. Convection usually calls to the case of fluids, if transmission of heat develops with movement and mixing of fluid involve conduction and if the fluid is transparent, radiation [1]. The most common biomass dryers use the principle of convection [11]. The fundamental variables both dependent and independent of the drying process are: temperatures in every period, duration of the drying process, consumption of energy and most of all efficiency of the drying process, i.e. final moisture with respect to the initial moisture of the biomass. They are much affected by the type of biomass to desiccate in every period during drying process, while the difficulties associated with the liberation of the retained water occur in every period [1]. If in a wood drying process the moisture diffusivity and moisture transfer coefficient are well determined, the good-quality dried products and energy savings in the process are attained; it is evident that decreasing energy consumption in the drying process will decrease the environmental impact in terms of pollutants, hence will protect the environment [13].
Thermal analysis is convenient and reproducible, and is a useful method for characterizing heterogeneous organic materials [14]. In past decades, differential thermal analyses (DTA), a differential scanning calorimetry (DSC) and thermogravimetric analysis (TG) have been used for analysing biological materials from plants [15, 16]. Wood is a natural composite polymeric material consisting of a cellulose network bonded together by rigid fixed polyosic lingo-polysaccharide amorphous matrix chains. The mechanical and chemical properties of wood are depicted by certain parameters such as density, stiffness and strength, which are closely related to the ultra-fine cell wall structure (less than 1 Å) [17–19]. Wood is considered to be a durable material, which withstands weathering without losing its structural properties. However, there are some non-biological parameters such as humidity, temperature, solar irradiation, ozone content and pollutants [17, 20] that may be responsible for the degradation of wood. Generally, the degradation of wood is caused by light irradiation or by heat treatment [17, 21, 22]. Thermal degradation of wood is highly dependent on its constituents [17, 23, 24].
In the present study, we have investigated the possibility of use of differential scanning calorimetry (DSC) and FT-IR spectroscopy for water—characterization of different kinds of wood biomass. Consequently, DSC curves and infrared spectra registered in the classic MIR range were used to monitor studied samples due to their water content before and after drying process.
Materials and methods
Materials
Sawdust of spruce (Picea abies), beech (Fagus sylvatica), willow (Salix alba) and alder (Alnus glutinosa) was studied. The woody biomass was obtained from a sawmill. It was assumed that each sample contains different amounts of regular wood components, e.g. cellulose, hemicellulose, lignin and water, in different percentage abundances. The wood particle size distribution was evaluated through screen analysis of the dried material. Sawdust particles totalling 86 % for spruce, 82 % for beech, 84 % for willow and 77 % for alder passed through the 10-mm screen and were stuck in the 0.5-mm screen. DSC and FT-IR spectroscopy were used for characterization of wood biomass before and after drying process. All experiments and analyses were carried out in triplicate (n = 3) for each sample, and the analytical data were used for statistical comparisons.
The drying experiments
The study of features of raw material included determination of the initial moisture level by oven-drying method and its change during entire drying process. The initial and final MC of sawdust was determined using the oven method at 105 °C for 72 h. The drying experiments were carried out using laboratory dryer Memmert UFP400 (Memmert GmbH +Co.KG, Schwabach, Germany). The change in MC during entire drying process was determined of sawdust of wood dried in a convective dryer at the temperature of 80 °C, without the flow of drying agent. Measurements of the MC changes carried out in the laboratory dryer Memmert UFP400 were taken in the following way. The sample was put on the tulle stretched on the metal frame (scale) and hung up to the electronic scales WPX 650 (Radwag, Radom, Poland). The accuracy of the weighing was ±0.001 g. Computer connected to the scales was an additional equipment of experimental stand. It recorded mass of dried sample at regular intervals of 1 min. Measurements of mass changes were recorded up to the moment when mass replicated three times. On the basis of outcomes of the carried out experiments, the drying curves were drawn.
DSC measurements
The differential scanning calorimeter (TA Instruments Q 200) with a normal-pressure DSC cell equipped with a cooling (N2) system was used to determine thermal properties of wood biomass before and after drying process. The DSC measurement was taken at the temperature range 50–500 °C. Nitrogen flow rate of 50 mL min−1 was set for the analysis. The calorimetric data were obtained with the wood sample enclosed in a covered aluminium pan with an empty pan considered reference.
FT-IR spectroscopy
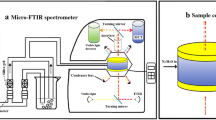
FT-IR spectroscopy was applied to measure absorption of energy from MIR range, e.g. 400–4000 cm−1 by studied samples. PerkinElmer System 200 spectrometer was applied with resolution set on 1 cm−1. Instrument was up-regulated by PC running on Windows 98 platform equipped with GRAMS/AI software allowing registration and processing of spectra and spectral data. Spectral registration was conducted with use of solid-state pallets made of potassium bromide used as a matrix thoroughly mixed with studied sample in laboratory ball mill to obtain fine powder. The ratio was approximately 1:300 mg. Powder was pressed in laboratory press with 10 tons pressure in dedicated pellet maker for 1–2 min. Obtained pellets were of 13 mm in diameter. For each sample, 15 spectra were registered and averaged. Water content was then related to integrate absorption in the region where water absorbs energy by O–H stretching vibrations that is 3000–3700 cm−1.
Results and discussion
Wood is a complex organic material, composed of cellulose (40–45 % for coniferous trees and 38–50 % for leafy trees), lignin (26–34 % for coniferous trees and 23–30 % for leafy trees), hemicelluloses (7–15 % for coniferous trees and 19–26 % for leafy trees), extractives (<15 %), ashes (<1 %) water and mineral matter. The chemical composition varies from species to species, and within the same variety, it varies with the botanical origin, age and location in the part of the tree, e.g. trunk, branches, crown and roots [25].
Thermal analysis is convenient and reproducible, and is a useful method for characterizing heterogeneous organic materials like biomass from wood. In past decades, differential thermal analyses (DTA) and a differential scanning calorimetry (DSC) have been used to analyse biological materials from plants. Cellulose, hemicelluloses and lignin, that are components of cells of plant walls, have been analysed by DTA and DSC for their thermal response [15]. The degradation of wood determined by DSC analysis provides information on the interaction between wood components and modification of their chemical structure upon heat treatment [17]. In current investigation, DSC curves of wood biomass samples have been analysed. A DSC response from the sawdust of wood biomass samples after drying process is shown in Fig. 1. Analysis of all type of wood biomass indicated three main exotherms. Maximum of exotherms are at ca. 280–310 °C, ca. 355–365 °C and ca. 410–430 °C, respectively. As the main components of wood are cellulose, hemicelluloses and lignin [26, 27], it can be concluded that obtained exotherms refer to the thermal decomposition of mentioned components and inform about interactions occurring between them. Position of DSC curve maximum of pure hemicellulose sample is according to the literature data [28] located at 268 °C, while data obtained in current study locate it at over 280 °C. This is an evidence that mentioned peak refers to hemicelluloses (similar location), but its shift toward higher temperature evidences that intermolecular forces between hemicelluloses molecules and another molecules exist and are stronger in the case of hemicelluloses present in wood than in the case of hemicelluloses molecules itself (pure hemicelluloses). Those additional and stronger forces are due to interaction of hemicelluloses molecules with another molecules, e.g. cellulose or lignin molecules. This interaction results in offset temperature. The same explanation for higher temperature maximum peaks as compared to the literature (pure) compounds can be referred to both lignin and cellulose.
Cellulose is the structural component of the primary cell wall of green plants. It is a polysaccharide consisting of a linear chain of several hundreds to over 10,000 linked d-glucose units. Unlike cellulose, hemicellulose (also a polysaccharide) consists of shorter chains: 500–3000 sugar units. Lignin is a three-dimensional polymer with phenylpropane as basic unit, linked by C–O–C and C–C bonds. Lignin is covalently linked to hemicellulose and, therefore, crosslinks different plant polysaccharides, conferring mechanical strength to the cell wall [29].
The literature data report that hemicellulose thermal degradation starts at around 180 °C and finishes at around 350 °C. Cellulose is comparatively more stable than hemicellulose; the temperature of its decomposition ranges from 200 °C to 375 °C, while lignin behaves differently [30]. According to Young et al. 2007 [28], thermal decomposition regions of hemicellulose and cellulose are in ranges of around 220–315 and 315–400 °C, respectively, with maximum mass losses at 268 and 355 °C. Lignin decomposition is in the range from 180 °C up to 900 °C with an undefined maximum mass loss. As was reported in [30], the three main components of wood namely: cellulose, lignin and hemicellulose decompose in temperatures ranging between 240–350, 280–500 and 200–260 °C, respectively. Considering the literature data, it can be concluded that hemicellulose is the least thermostabile and lignin is the most thermostable components of the three main components of wood. Analysing DSC curves of the samples which are the subject of research, it can be seen that in temperatures ranging between 250 and 330 °C, characteristic for hemicelluloses, the largest exotherm has beech biomass and the smallest has spruce biomass. In temperatures ranging between 330 and 370 °C, characteristic for celluloses, the largest exotherm for beech biomass and the smallest for spruce biomass were observed. Considering the temperatures ranging above 370 °C of analysed DSC curves may be noticed that largest exotherm for spruce biomass and the smallest for beech biomass appeared. Beech belongs to hardwoods and spruces to softwoods. General softwoods contain less cellulose and hemicelluloses, and more lignin. Cellulose represents about 40 and 60 % in the overall mass of dry wood (it accounts for 23–33 % of the mass of softwoods), 23–33 % of lignin in softwoods (16–25 % in hardwoods) and 25–35 % of hemicelluloses (more in hardwoods than in softwoods) [30]. The proportion of each wood component varies depending on the species, to a greater or lesser extent. Therefore, it is expected that the DSC curves are different depending on the type of wood to which they belong. As suggested by researchers [30], differences in the thermal degradation of lignin and hemicellulose depending on whether these come from softwood or hardwood, or even of different species, were observed. These results suggest that the curves obtained by differential scanning calorimetry (DSC) can be used as a discriminant characteristic.
One of the main non-combustible components of wood biomass is water. MC of green biomass or raw biomass materials (wood, bark, plants, etc.) commonly exceeds 50 mass% wb [31]. The initial MC (Fig. 2) of analysed samples was ranged from 0.71 to 0.75 kg H2O kg−1 dm for spruce (42.2 ± 0.9 % wb), 0.87–0.95 kg H2O kg−1 dm for beech (47.6 ± 1.6 % wb), 0.80–0.88 kg H2O kg−1 dm for willow (45.7 ± 1.7 % wb) and 1.04–1.16 kg H2O kg−1 dm for alder (52.4 ± 1.9 % wb). The presence of water has many negative effects on the net energy density and efficiency of biomass utilization, as well as on the performance of biomass liquid products. In such cases, many pyrolytic reactors of biomass are designed to operate on very low moisture-content material. Therefore, drying pretreatment of biomass material is usually desirable and sometimes essential to minimize the effects of moisture on biomass pyrolysis utilization [32]. In synthesis gas production the feedstocks must be dried to below the 30 % MC, preferably to about 15 %, and in pyrolysis to below 10 % [33]. Production of secondary standardized solid biofuels with lower MC and higher calorific value, such as wood pellets, also requires drying of raw wood chips/sawdust prior to pellet manufacturing. Wooden material for wood pellets production must have about 10 mass% wb [31]. The heat needed to evaporate 1 kg of water from moist (wet) biomass can exceed 2.6 MJ kg−1 (depending on initial and final MC and temperature of drying); therefore, drying is an energy-intensive process [11, 31]. For this reason that the drying process requires a lot of energy, it is important that it is performed in an optimal way [12]. The quality and properties of biofuels from wood depend among others on drying technology and parameters of drying process. Results of the drying experiments are illustrated in Fig. 3, which shows the drying curves. The figures show that the drying rate rose sharply at first, but dropped rapidly afterwards and slowly approached zero. The drying process is usually divided into two different drying regimes: the period of “constant drying rate”, where the process is determined by external conditions, and the period of “falling drying rate”, where the internal moisture migration limits the drying rate [12]. The moisture in raw materials can be classified as free moisture (bound to the biomass particle surface and existing in pores) and bound moisture (bound to the inner pores of biomass particles). These different binding forces between moisture and biomass led to different drying periods [32]. For samples being the subject of research (Fig. 3), we can also observe that in first period of drying process, the surface moisture continually evaporated a large amount of free moisture, which had weak binding force, escaped in the form of water vapour. Subsequently, with insufficient free moisture on the surface, the bound moisture became involved in the drying process. The drying rate was then dominated by diffusion from the inside to the surface of materials. Therefore, the drying rate started to slowly decline and approached zero. Analysing the drying curves can be seen that after 4-h drying at 80 °C almost all kinds of wood biomass, characterized by adequate moisture needed for the next stage of solid biofuel production. Only sawdust of alder contains more than 10 % moisture. The fastest desired MC on sawdust of spruce chips was obtained. The MC after 4 h of drying process at 80 °C of analysed samples (Fig. 2) were ca. 0.050 ± 0.004 kg H2O kg−1 dm for spruce (4.8 ± 0.4 % wb), 0.080 ± 0.009 kg H2O kg−1 dm for beech (7.4 ± 0.8 % wb), 0.061 ± 0.006 kg H2O kg−1 dm for willow (5.7 ± 0.5 % wb) and ca. 0.161 ± 0.006 kg H2O kg−1 dm for alder (13.8 ± 0.5 % wb).
Therefore, the higher biomass MC generally causes operational problems of biomass combustors, lower stability of burning and higher CO and VOC emissions; it is necessary to monitoring MC in the biomass for combustion and for biofuels production. Both CO and VOC are considered local environment pollutants, and their decrease is required due to environment protection. The characterization of the gaseous emissions is required as a crucial investigation firstly to evaluate the environmental and human pollution. Water contained in fuel facilitates some additional combustion chemical reactions that result in production of undesired compounds [31, 34]. Traditional methods such as oven-drying methods are long lasting. This paper attempts to differentiate studied samples due to water content before and after drying process by calorimetric and spectrometric methods. There are significant differences within courses of DSC curves of woody biomass before and after drying process (Fig. 4). In the temperature range of 80–120 °C, a characteristic broad band is observed. This endothermic peak with maximum at about 105 °C is assigned to water evaporation.
The water phase evaporated in this temperature range is called external water or bulk water, which is presented in large pores [29]. In all the samples before the drying process, a large endothermic peak derived from the water was observed, while in the samples after drying, the peak is very small. This confirms the presence of certain amount of humidity in the samples after 4 h of drying process. Designated enthalpy of phase transitions puts the peak area appearing in the temperature range of 80–120 °C for the biomass obtained after the process of drying amounted from ca. 70–100 kJ kg−1. Heat requirements of water evaporation from wood biomass before drying process can also be obtained by integrating the DSC curves. The calculation results indicated that water evaporation from biomass of spruce, beech, willow and alder required 467 ± 27, 789 ± 76, 558 ± 93 and 865 ± 41 kJ kg−1 of heat, respectively. Obtained values of enthalpy can be integrated with the MC in the wood biomass. Statistically significant (p value <0.05) correlations were found for MC and obtained values of enthalpy (Fig. 5). The values of R 2 (coefficient of determination)—0.9653—indicated that there is strong relation between those parameters. Results obtained are consistent with the value of enthalpy of evaporation of water (literature) that is 2257 kJ/kg (under standard pressure, i.e. 1 atm.). It is an amount of heat required to convert 1 kg of water into vapour being a function of pressure. In a physical model given by Garai [33], it is considered the energy equal to surface resistance of liquid—the energy molecule requires to change from liquid to gas phase. The relation established will be subject of detailed analysis in the future investigation with higher number of samples.
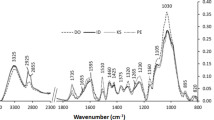
The spectra of studied samples are similar to each other within entire course; that is, similar absorption occurs in the same spectral regions. Significant wide absorption band is observed within 3700–3000 cm−1 region. This absorption is solely originated from vibrations of water present in samples. Characteristic very strong band occurring at 1616 cm−1 is associated with less intense also characteristic band at 1598 cm−1. This system of bands occurs in all samples. Another characteristic system of two bands occurs at 1372 and 1351 cm−1. It is again present in all studied samples. In the case of both two-band systems, mutual intensities of bands are the same; that is, band at 1616 cm−1 is more intense than one at 1598 cm−1, while in another pair band at 1372 cm−1 is more intense than band at 1351 cm−1. Mentioned similarities are due to similar general chemical composition. Small differences in spectral region below 1300 cm−1 are most probably due to different detailed chemical compositions of studied samples. Most significant differences in spectra of studied tree samples were observed in bands intensities but locations. Due to the aim of this study, water content was analysed. Spectra registered for all samples after drying process contained band characteristic for water absorption significantly less intense as compared to adequate band in samples before drying. This observation was expected and natural, while quantitative relation with water was not certain. Visual inspection of absorption spectra proved partially only that absorption within this spectral region differs between the same samples after and before drying in the case of all tree samples. Figure 6 presents spectra of beech before and after drying, e.g. continuous and dotted lines, respectively. Band intensity was calculated with use of GRAMS/AI software. This was followed by detailed qualitative statistical approach to correlate water content measured by standard oven-drying method with band intensities. Detailed data indicating correlation between two data sets are presented in Table 1. The best-fit line is y = 0.7286x − 41.04 with R 2 = 0.91. It is to note significant determinant coefficient of 0.9518 indicating statistically significant correlation between those data sets.
Conclusions
The calorimetric data provide detailed information on change in water content of woody biomass occurring on heat treatment. Presented results strongly suggest that the curves registered with differential scanning calorimetry (DSC) can be an effective tool for biomass samples discrimination due to water content. Interpretation of drying curves proved that after 4-h drying at 80 °C, samples of all kinds of wood biomass except alder contained water at the level allowing pass to the next stage of solid biofuel production. The exceptional sawdust of alder contained more than 10 % water. There are significant differences within courses of DSC curves of woody biomass measured before and after drying process (see “Result and discussion” section).
In all samples before drying process, a large endothermic peak generated by water evaporation was observed, while in the samples after drying, adequate peak was fairly small, which indicates sample dryness. Directly proportional correlation between measured values of enthalpy of water evaporation and water content in wood biomass was worked out and presented (see Fig. 5). Obtained spectral data also suggest possibility for quantitative correlation of content of water with IR data of studied sample (see Table 1). It is very important that results presented refer to studied biomass samples exclusively. As wood samples are of biological origin and may therefore differ significantly from each other due to qualitative and quantitative composition, results obtained in current study cannot be extended to another wood type at the moment.
Abbreviations
- wb:
-
Wet basis
- MC:
-
Moisture content
References
Gonzales JF, Ledesma B, Alkassir A, Gonzalez J. Study of the influence of the composition of several biomass pellets on the drying process. Biomass Bioenerg. 2011;35:4399–406.
Fantozzi F, Buratti C. Life cycle assessment of biomass chains: wood pellet from short rotation coppice using data measured on a real plant. Biomass Bioenerg. 2010;34:1796–804.
Eriksson HM, Hall JP, Helynen S. Rationale for forest energy production. In: Richardson J, Björheden R, Hakkila P, Lowe AT, Smith CT, editors. Bioenergy from sustainable forestry guiding principles and practice. New York: Kluwer Academic Publishers; 2002. p. 1–15.
Uran V. A model for establishing a win–win relationship between a wood pellets manufacturer and its customers. Biomass Bioenerg. 2010;34:747–53.
Demirbaş A. Biomass resource facilities and biomass conversion processing for fuels and chemicals. Energy Convers Manage. 2001;42:1357–78.
Zhang L, Xu C, Champagne P. Overview of recent advances in thermo—chemical conversion of biomass. Energy Convers Manage. 2010;51:969–82.
Demirbaş A. Waste management, waste resource facilities and waste conversion process. Energy Convers Manage. 2011;52:1280–7.
Febrero L, Granada E, Pérez C, Patiño D, Arce E. Characterisation and comparison of biomass ashes with different thermal histories using TG-DSC. J Therm Anal Calorim. 2014;118:669–80.
Bedane AH, Afzal MT, Sokhansanj S. Simulation of temperature and moisture changes during storage of woody biomass owing to weather variability. Biomass Bioenerg. 2011;35:3147–51.
Fyhr C, Rasmusun A. Mathematical model of steam chips and other hygroscopic drying of wood porous media. AIChE J. 1996;42(6):2491–502.
Selivanos J, Blumberga D, Ziemele J, Blumberga A, Barisa A. Research of woody biomass drying process in pellet production. Environ Clim Technol. 2012;10:46–51.
Johansson A, Fyhr C, Rasmuson A. High temperature convective drying of wood chips with air and superheated steam. Int J Heat Mass Transf. 1997;40(12):2843–58.
Dincer I. Moisture loss from wood products during drying—part I: moisture diffusivities and moisture transfer coefficients. Energ Source. 1998;20:67–75.
Bryś J, Wirkowska M, Górska A, Ostrowska-Ligęza E, Bryś A. Application of the calorimetric and spectroscopic methods in analytical evaluation of the human milk fat substitutes. J Therm Anal Calorim. 2014;118:841–8.
Tsujiyama S, Miyamori A. Assignment of DSC thermograms of wood and its components. Thermochim Acta. 2000;351:177–81.
Chetehouna K, Belayachi N, Rengel B, Hoxha D, Gillard P. Investigation on the thermal degradation and kinetic parameters of innovative insulation materials using TGA-MS. Appl Therm Eng. 2015;81:177–84.
Mehrotra R, Singh P, Kandpal H. Near infrared spectroscopic investigation of the thermal degradation of wood. Thermochim Acta. 2010;507–508:60–5.
Tsuchikawa S, Yonenobu H, Siesler HW. Near Infrared Spectroscopic observations of the aging process in archaeological wood using a deuterium exchange method. Analyst. 2005;130:379–84.
Åkerholm M. Salmén L Interactions between wood polymers studied by dynamic FT-IR spectroscopy. Polymer. 2001;42(3):963–9.
Colom X, Carrillo F, Nogues F, Garriga P. Structural analysis of photodegraded wood by means of FTIR spectroscopy. Polym Degrad Stabil. 2003;80:543–9.
Sandu ICA, Brebu M, Luca C, Sandu I, Vasile C. Thermogravimetric study on the ageing of lime wood supports of old paintings. Polym Degrad Stabil. 2003;80:83–91.
Esteves BM, Domingos IJ, Pereira HM. Pine wood modification by heat treatment in air. Bioresources. 2008;3(1):142–54.
Liu Q, Yang CLVY, He F, Ling L. Study on the pyrolysis of wood-derived rayon fiber by thermogravimetry–mass spectrometry. J Mol Struct. 2005;733:193–202.
Lee HL, Chen GC, Rowel RM. Thermal properties of wood reacted with a phosphorus pentoxide–amine system. J Appl Polym Sci. 2004;91:2465–81.
Leroy V, Cancellieri D, Leoni E. Relation between forest fuels composition and energy emitted during their thermal degradation. J Therm Anal Calorim. 2009;96:293–300.
Mattos BD, de Cademartori PHG, Magalhães WLE, Lazzarotto M, Gatto DA. Thermal tools in the evaluation of decayed and weathered wood polymer composites prepared by in situ polymerization. J Therm Anal Calorim. 2015;121:1263–71.
Maryandyshev P, Chernov A, Lyubov V, Trouve G, Brillard A, Brilhac JF. Investigation of thermal degradation of different wood-based biofuels of the northwest region of the Russian Federation. J Therm Anal Calorim. 2015;122:963–73.
Yang H, Yan R, Chen H, Lee DH, Zheng C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel. 2007;86:1781–8.
Su Y, Luo Y, Wu W, Zhang Y, Zhao S. Characteristics of pine wood oxidative pyrolysis: degradation behavior, carbon oxide production and head properties. J Anal Appl Pyrol. 2012;98:137–43.
Tarrio-Saavedra J, Naya S, Francisco-Fernandez M, Lopez-Beceiro J, Artiaga R. Functional nonparametric of wood species from thermal data. J Therm Anal Calorim. 2011;104:87–100.
Svoboda K, Martinec J, Pohorely M, Baxter D. Integration of biomass drying with combustion/gasification technologies and minimization of emissions of organic compounds. Chem Pap. 2009;63(1):15–25.
Chen D, Li M, Zhu X. TG – DSC method applied to drying characteristics and heat requirement of cotton stalk during drying. Heat Mass Transf. 2012;48:2087–94.
Garai J. Physical model for vaporization. Fluid Phase Equilibr. 2009;283:89–92.
Chetehouna K, Belayachi N, Lemée L, Hoxha D, Rengel B. Pyrolysis gases released during the thermal degradation of insulation materials based on straw fibers. J Therm Anal Calorim. 2015;122:1417–22.
Acknowledgements
The results of this research were presented at the CCTA conference 2015.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bryś, A., Bryś, J., Ostrowska-Ligęza, E. et al. Wood biomass characterization by DSC or FT-IR spectroscopy. J Therm Anal Calorim 126, 27–35 (2016). https://doi.org/10.1007/s10973-016-5713-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5713-2