Abstract
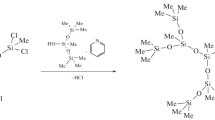
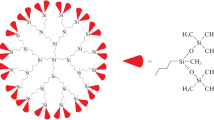
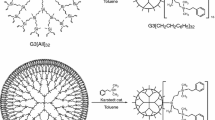
The molar heat capacity of siloxane dendrimer of the third generation with trimethylsilyl terminal groups G3[OSi(CH3)3]24 was determined by precise adiabatic calorimetry and differential scanning calorimetry over the temperature range T = (6–570) K for the first time. The low-temperature structural anomaly and the glass transition were observed in the above temperature range, and the standard thermodynamic characteristics of the revealed transformations were determined and analyzed. The fundamental thermodynamic functions such as the enthalpy [H°(T) − H°(0)], the entropy [S°(T) − S°(0)], and the Gibbs energy [G°(T) − H°(0)] were calculated for the range from T → 0 to 570 K based on the experimentally determined molar heat capacity of the investigated compound. The standard entropy of formation ΔfS° of dendrimer G3[OSi(CH3)3]24 was evaluated at T = 298.15 K. The thermal stability of the studied compound was investigated by thermogravimetric analysis. The standard thermodynamic properties of siloxane dendrimer G3[OSi(CH3)3]24 were compared and discussed with the previously reported data for the studied G3 carbosilane dendrimers with different functional terminal groups on the surface layer.


Similar content being viewed by others
References
Fréchet JMJ, Tomalia DA. Dendrimers and other dendritic polymers. Chichester: Wiley; 2001. https://doi.org/10.1002/0470845821.
Newkome GR, Moorefield CN, Vögtle F. Dendrimers and dendrons: concepts, syntheses, applications. Weinheim: Wiley; 2001. https://doi.org/10.1002/3527600612.
Muzafarov AM, Vasilenko NG, Tatarinova EA, Ignat’eva GM, Myakushev VM, Obrezkova MA, Meshkov IB, Voronina NV, Novozhilov OV. Macromolecular nano-objects as a promising direction of polymer chemistry. Polym Sci Ser C. 2011;53:48–60. https://doi.org/10.1134/S1811238211070022.
Buhleier E, Wehner W, Vögtle F. “Cascade”- and “nonskid-chain-like” syntheses of molecular cavity topologies. Synthesis. 1978;1978:155–8. https://doi.org/10.1055/s-1978-24702.
Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, Roeck J, Ryder J, Smith P. A new class of polymers: starburst-dendritic macromolecules. Polym J. 1985;17:117–32. https://doi.org/10.1295/polymj.17.117.
Newkome GR, Yao Z, Baker GR, Gupta VK. Micelles. Part 1. Cascade molecules: a new approach to micelles. A [27]-arborol. J Org Chem. 1985;50:2003–4. https://doi.org/10.1021/jo00211a052.
Hawker CJ, Fréchet JMJ. Preparation of polymers with controlled molecular architecture. A new convergent approach to dendritic macromolecules. J Am Chem Soc. 1990;112:7638–47. https://doi.org/10.1021/ja00177a027.
Rebrov EA, Muzafarov AM, Papkov VS, Zhdanov AA. Volume-growing polyorganosiloxanes. Proc USSR Acad Sci. 1989;309:376–80.
Muzafarov AM, Rebrov EA, Papkov VS. Three-dimensionally growing polyorganosiloxanes. Possibilities of molecular construction in highly functional systems. Russ Chem Rev. 1991;60:807–14. https://doi.org/10.1070/RC1991v060n07ABEH001112.
Morikawa A, Kakimoto M, Imai Y. Synthesis and characterization of new polysiloxane starburst polymers. Macromolecules. 1991;24:3469–74. https://doi.org/10.1021/ma00012a001.
Muzafarov AM, Tatarinova EA, Vasilenko NG, Ignat’eva GM. Organosilicon dendrimers and irregular hyperbranched polymers. In: Lee VY, editor. Organosilicon compounds: experiment (physico-chemical studies) and applications. Cambridge: Academic Press; 2017. p. 323–82. https://doi.org/10.1016/B978-0-12-814213-4.00008-3.
Lang H, Lühmann B. Siloxane and carbosiloxane based dendrimers: synthesis, reaction chemistry, and potential applications. Adv Mater. 2001;13:1523–40. https://doi.org/10.1002/1521-4095(200110)13:20%3C1523:AID-ADMA1523%3E3.0.CO;2-P.
Astruc D, Boisselier E, Ornelas C. Dendrimers designed for functions: from physical, photophysical, and supramolecular properties to applications in sensing, catalysis, molecular electronics, photonics, and nanomedicine. Chem Rev. 2010;110:1857–959. https://doi.org/10.1021/cr900327d.
Svenson S, Tomalia DA. Dendrimers in biomedical applications—reflections on the field. Adv Drug Deliv Rev. 2012;64:102–15. https://doi.org/10.1016/j.addr.2012.09.030.
Yang J, Zhang Q, Chang H, Cheng Y. Surface-engineered dendrimers in gene delivery. Chem Rev. 2015;115:5274–300. https://doi.org/10.1021/cr500542t.
Liko F, Hindré F, Fernandez-Megia E. Dendrimers as innovative radiopharmaceuticals in cancer radionanotherapy. Biomacromolecules. 2016;17:3103–14. https://doi.org/10.1021/acs.biomac.6b00929.
Lebedev BV, Ryabkov MV, Tatarinova EA, Rebrov EA, Muzafarov AM. Thermodynamic properties of the first to fifth generations of carbosilane dendrimers with allyl terminal groups. Russ Chem Bull. 2003;52:545–51. https://doi.org/10.1023/A:1023977916394.
Smirnova NN, Stepanova OV, Bykova TA, Markin AV, Muzafarov AM, Tatarinova EA, Myakushev VD. Thermodynamic properties of carbosilane dendrimers of the third to the sixth generations with terminal butyl groups in the range from T → 0 to 600 K. Thermochim Acta. 2006;440:188–94. https://doi.org/10.1016/j.tca.2005.11.009.
Markin AV, YaS Samosudova, Smirnova NN, Tatarinova EA, Bystrova AV, Muzafarov AM. Thermodynamics of carbosilane dendrimers with diundecylsilyl and diundecylsiloxane terminal groups. J Therm Anal Calorim. 2011;105:663–76. https://doi.org/10.1007/s10973-010-1199-5.
Smirnova NN, Markin AV, Samosudova YS, Ignat’eva GM, Katarzhnova EY, Muzafarov AM. Thermodynamics of G-3(D4) and G-6(D4) carbosilanecyclosiloxane dendrimers. Russ J Phys Chem A. 2013;87:552–9. https://doi.org/10.1134/S0036024413040262.
Smirnova NN, Markin AV, Letyanina IA, Sologubov SS, Novozhilova NA, Tatarinova EA, Muzafarov AM. Thermodynamic properties of carbosilane dendrimers of the third and sixth generations with ethyleneoxide terminal groups. Russ J Phys Chem A. 2014;88:735–41. https://doi.org/10.1134/S0036024414050306.
Markin AV, Sologubov SS, Smirnova NN, Knyazev AV, Mączka M, Ptak M, Novozhilova NA, Tatarinova EA, Muzafarov AM. Calorimetric and infrared studies of carbosilane dendrimers of the third generation with ethyleneoxide terminal groups. Thermochim Acta. 2015;617:144–51. https://doi.org/10.1016/j.tca.2015.08.028.
Sologubov SS, Markin AV, Smirnova NN, Novozhilova NA, Tatarinova EA, Muzafarov AM. Thermodynamic properties of carbosilane dendrimers of the sixth generation with ethylene oxide terminal groups. J Phys Chem B. 2015;119:14527–35. https://doi.org/10.1021/acs.jpcb.5b06786.
Sologubov SS, Markin AV, Smirnova NN, Rybakova YA, Novozhilova NA, Tatarinova EA, Muzafarov AM. Calorimetric study of carbosilane dendrimers of the third and sixth generations with phenylethyl terminal groups. J Therm Anal Calorim. 2016;125:595–606. https://doi.org/10.1007/s10973-016-5301-5.
Smirnova NN, Sologubov SS, Sarmini YA, Markin AV, Novozhilova NA, Tatarinova EA, Muzafarov AM. Thermodynamic properties of first- and third-generation carbosilane dendrimers with terminal phenyldioxolane groups. Russ J Phys Chem A. 2017;91:2317–25. https://doi.org/10.1134/S0036024417110279.
Sologubov SS, Markin AV, Smirnova NN, Novozhilova NA, Tatarinova EA, Muzafarov AM. Thermodynamic properties of a first-generation carbosilane dendrimer with terminal phenylethyl groups. Russ J Phys Chem A. 2018;92:235–43. https://doi.org/10.1134/S0036024418010260.
Boldyrev K, Tatarinova E, Meshkov I, Vasilenko N, Buzin M, Novikov R, Vasil’ev V, Shtykova E, Feigin L, Bystrova A, Chvalun S, Muzafarov A. New approach to the synthesis of polymethylsilsesquioxane dendrimers. Polymer. 2019;174:159–69. https://doi.org/10.1016/j.polymer.2019.04.030.
Kurbatov AO, Balabaev NK, Mazo MA, Kramarenko EY. Molecular dynamics simulations of single siloxane dendrimers: molecular structure and intramolecular mobility of terminal groups. J Chem Phys. 2018;148:014902-1–-10. https://doi.org/10.1063/1.5009988.
Meija J, Coplen TB, Berglund M, Brand WA, De Bièvre P, Gröning M, Holden NE, Irrgeher J, Loss RD, Walczyk T, Prohaska T. Atomic weights of the elements 2013 (IUPAC technical report). Pure Appl Chem. 2016;88:265–91. https://doi.org/10.1515/pac-2015-0305.
Varushchenko RM, Druzhinina AI, Sorkin EL. Low-temperature heat capacity of 1-bromoperfluorooctane. J Chem Thermodyn. 1997;29:623–37. https://doi.org/10.1006/jcht.1996.0173.
Sabbah R, Xu-wu A, Chickos JS, Planas Leitão ML, Roux MV, Torres LA. Reference materials for calorimetry and differential thermal analysis. Thermochim Acta. 1999;331:93–204. https://doi.org/10.1016/S0040-6031(99)00009-X.
Höhne GWH, Hemminger WF, Flammersheim HJ. Differential scanning calorimetry. 2nd ed. Berlin: Springer; 2003. https://doi.org/10.1007/978-3-662-06710-9.
Drebushchak VA. Calibration coefficient of a heat-flow DSC. Part II. Optimal calibration procedure. J Therm Anal Calorim. 2005;79:213–8. https://doi.org/10.1007/s10973-004-0586-1.
Della Gatta G, Richardson MJ, Sarge SM, Stølen S. Standards, calibration, and guidelines in microcalorimetry. Part 2. Calibration standards for differential scanning calorimetry (IUPAC technical report). Pure Appl Chem. 2006;78:1455–76. https://doi.org/10.1351/pac200678071455.
Kaisersberger E, Janoschek J, Wassmer E. A heat flux DSC for enthalpy and specific heat determinations to 1700 K. Thermochim Acta. 1989;148:499–505. https://doi.org/10.1016/0040-6031(89)85253-0.
ASTM E1269-11. Standard test method for determining specific heat capacity by differential scanning calorimetry. West Conshohocken: ASTM International; 2018. https://astm.org/Standards/E1269.htm. Accessed 24 June 2019.
ISO 11357-4:2014. Plastics—differential scanning calorimetry (DSC)—part 4: determination of specific heat capacity. Geneva: International Organization for Standardization; 2014. https://iso.org/standard/65087.html. Accessed 24 June 2019.
Adam G, Gibbs JH. On the temperature dependence of cooperative relaxation properties in glass-forming liquids. J Chem Phys. 1965;43:139–46. https://doi.org/10.1063/1.1696442.
Kauzmann W. The nature of the glassy state and the behavior of liquids at low temperatures. Chem Rev. 1948;43:219–56. https://doi.org/10.1021/cr60135a002.
Bestul AB, Chang SS. Excess entropy at glass transformation. J Chem Phys. 1964;40:3731–3. https://doi.org/10.1063/1.1725086.
Wooley KL, Hawker CJ, Pochan JM, Fréchet JMJ. Physical properties of dendritic macromolecules: a study of glass transition temperature. Macromolecules. 1993;26:1514–9. https://doi.org/10.1021/ma00059a006.
Debye P. Zur Theorie der spezifischen Wärmen. Ann Phys. 1912;344:789–839. https://doi.org/10.1002/andp.19123441404.
McCullough JP, Scott DW. Experimental thermodynamics. Volume I. Calorimetry of non-reacting systems. London: Butterworth & Co., Ltd.; 1968.
Chase MW Jr. NIST-JANAF thermochemical tables. 4th ed. J Phys Chem Ref Data, Monograph No. 9. 1998;1–1951.
Acknowledgements
This work was performed with the financial support of the Ministry of Science and Higher Education of the Russian Federation (Contract No. 4.5706.2017/8.9), the Russian Foundation for Basic Research (Project No. 19-03-00248), and the Russian Science Foundation (Project No. 18-13-00411).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sologubov, S.S., Markin, A.V., Sarmini, Y.A. et al. Calorimetric study of siloxane dendrimer of the third generation with trimethylsilyl terminal groups. J Therm Anal Calorim 138, 3301–3310 (2019). https://doi.org/10.1007/s10973-019-08693-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08693-9