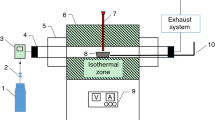
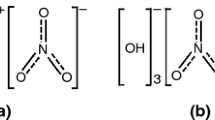
Abstract
The pyrolysis gas products of chlorinated polyvinyl chloride (CPVC) are first discussed based on thermogravimetry–Fourier transform infrared spectra–mass spectrometry (TG–FTIR–MS) analysis. The results of TG–FTIR preliminarily show that CPVC pyrolysis can be divided into two stages: the main gas products or functional groups are hydrogen chloride (HCl), chlorinated compounds, alkanes, alkenes and aromatic compounds in Stage I, while alkanes, aromatic compounds and alkenes in Stage II. By coupling MS, the main products can be further refined into hydrogen chloride and benzene in Stage I, while homologues, derivatives and polycyclic aromatic compounds of benzene (xylene and ethylbenzene, chlorobenzene and 1-chloronaphthalene, naphthalene and fluorene and so on) in Stage II. Moreover, compared with the pyrolysis gas products of PVC, chlorine is not completely converted into hydrogen chloride, and some of it is converted into other chlorinated compounds. Based on the above results, the possible reactions from molecular structure during CPVC pyrolysis are also put forward. Furthermore, the CPVC combustion properties and smoke production in cone calorimeter experiment are analyzed by the measured mass loss rate, heat release rate, smoke and CO/CO2 production rate.










Similar content being viewed by others
References
Dayana ASS, Abnisa F, Ashri WDWM, Kheireddine AM. A review on pyrolysis of plastic wastes. Energy Convers Manag. 2016;115:308–26. https://doi.org/10.1016/j.enconman.2016.02.037.
An W, Jiang L, Sun J, Liew KM. Correlation analysis of sample thickness, heat flux, and cone calorimetry test data of polystyrene foam. J Therm Anal Calorim. 2015;119:229–38. https://doi.org/10.1007/s10973-014-4165-9.
Yang Q, Lu W, Bai L, Yan B, Cheng Y. UV enhanced gas–solid synthesis of chlorinated poly vinyl chloride characterized by a UV–Vis online analysis method. Chin J Chem Eng. 2015;23(6):1052–9. https://doi.org/10.1016/j.cjche.2014.12.002.
Zhang W, Zhang J, Ding Y, Zhou R, Mao S. The accuracy of multiple methods for estimating the reaction order of representative thermoplastic polymers waste for energy utilization. Energy. 2022;239B:122112. https://doi.org/10.1016/j.energy.2021.122112.
Zhang W, Zhang J, Ding Y, He Q, Lu K, Chen H. Pyrolysis kinetics and reaction mechanism of expandable polystyrene by multiple kinetics methods. J Clean Prod. 2021;285: 125042. https://doi.org/10.1016/j.jclepro.2020.125042.
Huang J, Li X, Zeng G, Cheng X, Tong H, Wang D. Thermal decomposition mechanisms of poly(vinyl chloride): a computational study. Waste Manag. 2018;76:483–96. https://doi.org/10.1016/j.wasman.2018.03.033.
Wu J, Chen T, Luo X, Han D, Wang Z, Wu J. TG/FTIR analysis on co-pyrolysis behavior of PE, PVC and PS. Waste Manag. 2014;34(3):676–82. https://doi.org/10.1016/j.wasman.2013.12.005.
Jin W, Shen D, Liu Q, Xiao R. Evaluation of the co-pyrolysis of lignin with plastic polymers by TG–FTIR and Py-GC/MS. Polym Degrad Stab. 2016;133:65–74. https://doi.org/10.1016/j.polymdegradstab.2016.08.001.
Zhou R, Huang B, Ding Y, Li W, Mu J. Thermal decomposition mechanism and kinetics study of plastic waste chlorinated polyvinyl chloride. Polymers. 2019. https://doi.org/10.3390/polym11122080.
Ding Y, Ezekoye OA, Zhang J, Wang C, Lu S. The effect of chemical reaction kinetic parameters on the bench-scale pyrolysis of lignocellulosic biomass. Fuels. 2018;232:147–53. https://doi.org/10.1016/j.fuel.2018.05.140.
Ding Y, Huang B, Li K, Du W, Lu K, Zhang Y. Thermal interaction analysis of isolated hemicellulose and cellulose by kinetic parameters during biomass pyrolysis. Energy. 2020;195: 117010. https://doi.org/10.1016/j.energy.2020.117010.
Kai X, Li R, Yang T, Shen S, Ji Q, Zhang T. Study on the co-pyrolysis of rice straw and high density polyethylene blends using TG–FTIR–MS. Energy Convers Manag. 2017;146:20–33. https://doi.org/10.1016/j.enconman.2017.05.026.
Kai X, Yang T, Shen S, Li R. TG–FTIR–MS study of synergistic effects during co-pyrolysis of corn stalk and high-density polyethylene (HDPE). Energy Convers Manag. 2019;181:202–13. https://doi.org/10.1016/j.enconman.2018.11.065.
Chen R, Lu S, Zhang Y, Lo S. Pyrolysis study of waste cable hose with thermogravimetry/Fourier transform infrared/mass spectrometry analysis. Energy Convers Manag. 2017;153:83–92. https://doi.org/10.1016/j.enconman.2017.09.071.
Chen R, Xu X, Lu S, Zhang Y, Lo S. Pyrolysis study of waste phenolic fibre-reinforced plastic by thermogravimetry/Fourier transform infrared/mass spectrometry analysis. Energy Convers Manag. 2018;165:555–66. https://doi.org/10.1016/j.enconman.2018.03.092.
Gao X, Jiang L, Xu Q. Experimental and theoretical study on thermal kinetics and reactive mechanism of nitrocellulose pyrolysis by traditional multi kinetics and modeling reconstruction. J Hazard Mater. 2020;386: 121645. https://doi.org/10.1016/j.jhazmat.2019.121645.
Luche J, Rogaume T, Richard F, Guillaume E. Characterization of thermal properties and analysis of combustion behavior of PMMA in a cone calorimeter. Fire Saf J. 2011;46(7):451–61. https://doi.org/10.1016/j.firesaf.2011.07.005.
Luche J, Mathis E, Rogaume T, Richard F, Guillaume E. High-density polyethylene thermal degradation and gaseous compound evolution in a cone calorimeter. Fire Saf J. 2012;54:24–35. https://doi.org/10.1016/j.firesaf.2012.08.002.
Quang Dao D, Luche J, Richard F, Rogaume T, Bourhy-Weber C, Ruban S. Determination of characteristic parameters for the thermal decomposition of epoxy resin/carbon fibre composites in cone calorimeter. Int J Hydrog Energy. 2013;38(19):8167–78. https://doi.org/10.1016/j.ijhydene.2012.05.116.
Qu H, Liu C, Wu W, Chen L, Xu J. Using cone calorimeter to study thermal degradation of flexible PVC filled with zinc ferrite and Mg(OH)2. J Therm Anal Calorim. 2014;115:1081–7. https://doi.org/10.1007/s10973-013-3434-3.
Bai X, Wang Q, Sui S, Zhang C. The effects of wood-flour on combustion and thermal degradation behaviors of PVC in wood-flour/poly(vinyl chloride) composites. J Anal Appl Pyrolysis. 2011;91(1):34–9. https://doi.org/10.1016/j.jaap.2011.02.009.
IS0-5660-1. Reaction-to-fire tests-heat release, smoke production and mass loss rate—Part 1 heat release rate (cone calorimeter method). Switzerland; 2002.
Srivastav V, Sabarilal S, Kumar A. Numerical study on sample thickness dependence of fire response properties of polymeric materials (charring and non-charring) in standard cone calorimeter test. In: Gupta A, Mongia H, Chandna P, Sachdeva G, editors. Advances in IC engines and combustion technology. NCICEC 2019. Lecture notes in mechanical engineering. Singapore: Springer. https://doi.org/10.1007/978-981-15-5996-9_71.
Özsin G, Pütün AE. TGA/MS/FT-IR study for kinetic evaluation and evolved gas analysis of a biomass/PVC co-pyrolysis process. Energy Convers Manag. 2019;182:143–53. https://doi.org/10.1016/j.enconman.2018.12.060.
Xu F, Wang B, Yang D, Hao J, Qiao Y, Tian Y. Thermal degradation of typical plastics under high heating rate conditions by TG–FTIR: pyrolysis behaviors and kinetic analysis. Energy Convers Manag. 2018;171:1106–15. https://doi.org/10.1016/j.enconman.2018.06.047.
Soudais Y, Moga L, Blazek J, Lemort F. Coupled DTA–TGA–FT-IR investigation of pyrolytic decomposition of EVA, PVC and cellulose. J Anal Appl Pyrolysis. 2007;78(1):46–57. https://doi.org/10.1016/j.jaap.2006.04.005.
Zhu HM, Jiang XG, Yan JH, Chi Y, Cen KF. TG–FTIR analysis of PVC thermal degradation and HCl removal. J Anal Appl Pyrolysis. 2008;82(1):1–9. https://doi.org/10.1016/j.jaap.2007.11.011.
Zhou H, Meng A, Long Y, Li Q, Zhang Y. Interactions of municipal solid waste components during pyrolysis: a TG–FTIR study. J Anal Appl Pyrolysis. 2014;108:19–25. https://doi.org/10.1016/j.jaap.2014.05.024.
Sun Q, Shi X, Lin Y, Zhu H, Wang X, Cheng C, et al. Thermogravimetric–mass spectrometric study of the pyrolysis behavior of PVC. J China Univ Min Technol. 2007;17(2):242–5. https://doi.org/10.1016/s1006-1266(07)60080-7.
Knümann R, Bockhorn H. Investigation of the kinetics of pyrolysis of PVC by TG–MS-analysis. Combust Sci Technol. 1994;101(1–6):285–99. https://doi.org/10.1080/00102209408951877.
Huang L. Production and application of chlorinated PVC resins. Polyvinyl Chloride. 2002;1:15–20 (in Chinese).
Rhodes BT, Quintiere GJ. Burning rate and flame heat flux for PMMA in a cone calorimeter. Fire Saf J. 1996;26(3):221–40. https://doi.org/10.1016/S0379-7112(96)00025-2.
Chen R, Lu S, Li C, Ding Y, Zhang B, Lo S. Correlation analysis of heat flux and cone calorimeter test data of commercial flame-retardant ethylene–propylene-diene monomer (EPDM) rubber. J Therm Anal Calorim. 2015;123(1):545–56. https://doi.org/10.1007/s10973-015-4900-x.
Chen R, Xu X, Zhang Y, Lu S, Lo S. Characterization of ignition and combustion characteristics of phenolic fiber-reinforced plastic with different thicknesses. J Therm Anal Calorim. 2019;140(2):645–55. https://doi.org/10.1007/s10973-019-08903-4.
Ding Y, Fukumoto K, Ezekoye OA, Lu S, Wang C, Li C. Experimental and numerical simulation of multi-component combustion of typical charring material. Combust Flame. 2020;211:417–29. https://doi.org/10.1016/j.combustflame.2019.10.016.
Zhang Z, Wang C, Huang G, Liu H, Zhao W. Thermal decomposition characteristic parameters for the outer material of composite hydrogen storage tank by cone calorimeter. J Therm Anal Calorim. 2019;138(2):1299–310. https://doi.org/10.1007/s10973-019-08189-6.
Xu Z, Yan L, Liu Y. Study on Correlations between the flammability and dynamic smoke properties of four decorative materials. Procedia Eng. 2014;84:498–505. https://doi.org/10.1016/j.proeng.2014.10.461.
Acknowledgements
The authors would like to acknowledge financial support sponsored by National Natural Science Foundation of China (No. 51806202) and Jiangsu Key Laboratory of Hazardous Chemicals Safety and Control (No. HCSC201901).
Funding
National Natural Science Foundation of China, 51806202, Yanming Ding, Jiangsu Key Laboratory of Hazardous Chemicals Safety and Control, HCSC201901, Yanming Ding.
Author information
Authors and Affiliations
Contributions
AL and BH were involved in the methodology, validation, data curation, and writing. WZ contributed to the methodology, and writing. YD was involved in the supervision, funding acquisition, project administration, and writing. RZ contributed to the data curation.
Corresponding authors
Ethics declarations
Conflict of interest
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, A., Huang, B., Zhang, W. et al. Experimental study on pyrolysis gas products of chlorinated polyvinyl chloride and its smoke properties during combustion. J Therm Anal Calorim 147, 8213–8224 (2022). https://doi.org/10.1007/s10973-021-11156-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-021-11156-9