Abstract
Hypothalamus is the most critical center in the brain for regulation of food intake. Hypothalamus performs this function through special nuclei. The most important of these nuclei is PVN, because in addition to receiving input from special hypothalamic nuclei, it also receives input from other regions of the brain, as well circulation. Inputs received from other areas of the brain via special receptors including: MCR, GABA, IR, LepR, CBR, OXR, HR, NPY, D, CRF, and GHSR. Due to the presence of several receptors on VMH, different neurotransmitters and neuromodulators related to central food intake regulation effect on this nucleus. These neurotransmitters include two categories: orexigenic and anorexigenic. Orexigenic neuropeptides such as: NPY, orexin, endocannabinoids, glutamate, urocortin, and ghrelin. Anorexigenic neuropeptides included: MSH, CRF, leptin, insulin, BDNF, histamine, and dopamine. Then, VMH integrates these inputs from the bind of these neurotransmitters to their receptors, and sends the final feedback to other brain regions. The VMH is the first satiety center in the brain, and it receives various inputs from different regions of the brain and circulation via multiple receptors, as well as integrating these inputs and sending the appropriate output to other areas of the brain, VMH plays an important role in central control of food intake. Therefore, throughout this review article would discuss the function of this nucleus on central regulation of food intake via various neuropeptides and receptors.
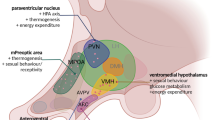
Graphic Abstract
Legend: ARC: Arcuate nucleus. PVN: Paraventricular nucleus. VMH: Ventromedial hypothalamus. SF1: Serotonergic factor 1. BDNF: Brain-derived neurotropic factor. POMC: pro-opiomelanocortin. AgRP/NPY: Agouti related protein/ Neuropeptide Y. VTA: Ventral tegmental area. CB: Endocannabinoidergic neuron. OX: Orexin neuron. TB: Tuberommillary nucleus. H: Histaminergic neuron. D: Dopaminergic neuron. G: Glutamatergic neuron.: Stimulatory projection.: Inhibitory projection.

Similar content being viewed by others
Abbreviations
- ARC:
-
Arcuate nucleus
- VMH:
-
Ventromedial hypothalamus
- PVN:
-
Paraventricular nucleus
- LHA:
-
Lateral hypothalamus area
- POMC:
-
Pro-opiomelanocortin
- CART:
-
Cocaine–amphetamine regulate transcript
- NPY:
-
Neuropeptide Y
- AgRP:
-
Agouti related protein
- MSH:
-
Melanocortin stimulating hormone
- CRF:
-
Corticotropin releasing factor
- BDNF:
-
Brain-derived neurotropic factor
- CNS:
-
Central nervous system
- MCR:
-
Melanocortin receptors
- CAMP:
-
Cyclic adenosine monophosphate
- DMH:
-
Dorsomedial nucleus
- SF1:
-
Steroidogenic factor 1
- GABA:
-
γ-Aminobutyric acid
- IR:
-
Insulin receptor
- LepR:
-
Leptin receptor
- CBR:
-
Endocannabinoid receptor
- MAPK:
-
Mitogen activated protein kinase
- OXR:
-
Orexin receptor
- HR:
-
Histaminergic receptor
- D:
-
Dopamine
- GHSR:
-
Growth hormone secretagogues receptor
- Vglut:
-
Vesicular glutamate transporter
- N/OFQ:
-
Nociceptin/orphanin FQ
- ORL-1:
-
Orphan Gi/Go-coupled opioid receptors
- GIRK:
-
G protein-coupled inwardly-rectifying potassium
- ICV:
-
Intracerebroventricular
- ACTH:
-
Adrenocorticotropin hormone
- VTA:
-
Ventral tegmental area
- BBB:
-
Blood brain barrier
References
Bäckberg M, Hervieu G, Wilson S, Meister B (2002) Orexin receptor-1 (OX-R1) immunoreactivity in chemically identified neurons of the hypothalamus: focus on orexin targets involved in control of food and water intake. Eur J Neurosci 15(2):315–328
Baghbanzadeh A, Babapour V (2007) Glutamate ionotropic and metabotropic receptors affect feed intake in broiler cockerels. IJVM 1(1):125–129
Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM (2005) Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123(3):493–505
Bariohay B, Roux J, Tardivel C, Trouslard J, Jean A, Lebrun B (2009) Brain-derived neurotrophic factor/tropomyosin-related kinase receptor type B signaling is a downstream effector of the brainstem melanocortin system in food intake control. Endocrinology 150(6):2646–2653
Bellocchio L, Soria-Gómez E, Quarta C, Metna-Laurent M, Cardinal P, Binder E, Cannich A, Delamarre A, Häring M, Martín-Fontecha M, Vega D (2013) Activation of the sympathetic nervous system mediates hypophagic and anxiety-like effects of CB1 receptor blockade. PANS 110(12):4786–4791
Betley JN, Cao ZF, Ritola KD, Sternson SM (2013) Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell 155(6):1337–1350
Blandina P, Provensi G, Munari L, Passani MB (2012) Histamine neurons in the tuberomamillary nucleus: a whole center or distinct subpopulations? Front Syst Neurosci 6:33
Cassaglia PA, Hermes SM, Aicher SA, Brooks VL (2011) Insulin acts in the arcuate nucleus to increase lumbar sympathetic nerve activity and baroreflex function in rats. J Physiol 589(7):1643–1662
Chao H, Digruccio M, Chen P, Li C (2012) Type 2 corticotropin-releasing factor receptor in the ventromedial nucleus of hypothalamus is critical in regulating feeding and lipid metabolism in white adipose tissue. Endocrinology 153:166–176
Chee MJ, Myers MG, Price CJ, Colmers WF (2010) Neuropeptide Y suppresses anorexigenic output from the ventromedial nucleus of the hypothalamus. J Neurosci 30(9):3380–3390
Chee MJ, Price CJ, Statnick MA, Colmers WF (2011) Nociceptin/orphanin FQ suppresses the excitability of neurons in the ventromedial nucleus of the hypothalamus. J Physiol 589(13):3103–3114
Chen P, Vaughan J, Donaldson C, Vale W, Li C (2010) Injection of Urocortin 3 into the ventromedial hypothalamus modulates feeding, blood glucose levels, and hypothalamic POMC gene expression but not the HPA axis. Am J Physiol Endocrinol Metab 298(2):337–345
Chen P, Van Hover C, Lindberg D, Li C (2013) Central urocortin 3 and type 2 corticotropin-releasing factor receptor in the regulation of energy homeostasis: critical involvement of the ventromedial hypothalamus. Front Endocrinol 3:180
Cheung CC, Krause WC, Edwards RH, Yang CF, Shah NM, Hnasko TS, Ingraham HA (2015) Sex-dependent changes in metabolism and behavior, as well as reduced anxiety after eliminating ventromedial hypothalamus excitatory output. Mol Metab 4(11):857–866
Chowdhury GM, Wang P, Ciardi A, Mamillapalli R, Johnson J, Zhu W, Eid T, Behar K, Chan O (2017) Impaired glutamatergic neurotransmission in the ventromedial hypothalamus may contribute to defective counterregulation in recurrently hypoglycemic rats. Diabetes 66(7):1979–1989
Cullen MJ, Ling N, Foster AC, Pelleymounter MA (2001) Urocortin, corticotropin releasing factor-2 receptors and energy balance. Endocrinology 142(3):992–999
Cummings DE (2006) Ghrelin and the short-and long-term regulation of appetite and body weight. Physiol Behav 89(1):71–84
De Backer MW, La Fleur SE, Brans MA, Van Rozen AJ, Luijendijk MC, Merkestein M, Garner KM, Van Der Zwaal EM, Adan RA (2011) Melanocortin receptor-mediated effects on obesity are distributed over specific hypothalamic regions. Int J Obes 5(5):629–641
Delgado TC (2013) Glutamate and GABA in appetite regulation. Front Endocrinol 4:103
Deng C, Weston-Green K, Huang XF (2010) The role of histaminergic H1 and H3 receptors in food intake: a mechanism for atypical antipsychotic-induced weight gain? Prog Neuro-Psychoph 34(1):1–4
Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R (2006) Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49(2):191–203
Faber CL, Matsen ME, Velasco KR, Damian V, Phan BA, Adam D, Therattil A, Schwartz MW, Morton GJ (2018) Distinct neuronal projections from the hypothalamic ventromedial nucleus mediate glycemic and behavioral effects. Diabetes 67(12):2518–2529
Fekete ÉM, Inoue K, Zhao Y, Rivier JE, Vale WW, Szücs A, Koob GF, Zorrilla EP (2007) Delayed satiety-like actions and altered feeding microstructure by a selective type 2 corticotropin-releasing factor agonist in rats: intra-hypothalamic urocortin 3 administration reduces food intake by prolonging the post-meal interval. Neuropsychopharmacology 32(5):1052–1068
Flanagan-Cato LM, Fluharty SJ, Weinreb EB, LaBelle DR (2008) Food restriction alters neuronal morphology in the hypothalamic ventromedial nucleus of male rats. Endocrinology 149(1):93–99
Fu LY, van den Pol AN (2008) Agouti-related peptide and MC3/4 receptor agonists both inhibit excitatory hypothalamic ventromedial nucleus neurons. J Neurosci 28(21):5433–5449
Gao Q, Horvath TL (2007) Neurobiology of feeding and energy expenditure. Annu Rev Neurosci 30:367–398
Gao Q, Horvath TL (2008) Neuronal control of energy homeostasis. FEBS Lett 582(1):132–1341
Gardiner JV, Bataveljic A, Patel NA, Bewick GA, Roy D, Campbell D, Greenwood HC, Murphy KG, Hameed S, Jethwa PH, Ebling FJ (2010) Prokineticin 2 is a hypothalamic neuropeptide that potently inhibits food intake. Diabetes 59(2):397–406
Ghamari-Langroudi M, Srisai D, Cone RD (2011) Multinodal regulation of the arcuate/paraventricular nucleus circuit by leptin. Proc Natl Acad Sci 108(1):355–360
Giannoni P, Passani MB, Nosi D, Chazot PL, Shenton FC, Medhurst AD, Munari L, Blandina P (2009) Heterogeneity of histaminergic neurons in the tuberomammillary nucleus of the rat. Eur J Neurosci 29(12):2363–2374
Gilland KE, Fox EA (2017) Effect of food deprivation or short-term Western diet feeding on BDNF protein expression in the hypothalamic arcuate, paraventricular, and ventromedial nuclei. Am J Physiol-Reg I 312(4):611–625
Hamidi F, Yousefvand S (2017) Role of the hypothalamic arcuate nucleus in regulation of food intake (review study). J Neyshabur Univ Med Sci 5(1):52–65 (Persian)
Harris RB (2010) Leptin responsiveness of mice deficient in corticotrophin-releasing hormone receptor type 2. Neuroendocrinology 92(3):198–206
Hasan TF, Hasan H (2011) Anorexia nervosa: a unified neurological perspective. Int J Med Sci 8(8):679
Hashiguchi H, Sheng Z, Routh V, Gerzanich V, Simard JM, Bryan J (2017) Direct versus indirect actions of ghrelin on hypothalamic NPY neurons. PloS one 12(9).
Hillebrand JJ, Kas MJ, Adan RA (2005) a-MSH enhances activity-based anorexia. Peptides 26(10):1690–1696
Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ (2006) Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 51(6):801–810
Ichijo A, Hayashi N, Fukuoka C, Hu JJ, Yoshizawa F, Sugahara K (2008) Dopamine release in the ventromedial hypothalamus of growing chickens decreases when they are fed a lysine devoid diet. Poult Sci 45(4):281–286
Iigaya K, Minoura Y, Onimaru H, Kotani S, Izumizaki M (2019) Effects of feeding-related peptides on neuronal oscillation in the ventromedial hypothalamus. Clin Med 8(3):292
Isganaitis E, Lustig RH (2005) Fast food, central nervous system insulin resistance, and obesity. Arterioscler Thromb Vasc Biol 25(12):2451–2462
Jaefari-Anari M, Zendehdel M, Gilanpour H, Asghari A, Babapour V (2018) Central opioidergic system interplay with histamine on food intake in neonatal chicks: role of µ-opioid and H1/H3 receptors. Braz J Poultry Sci 20(3):595–604
Jelsing J, Larsen PJ, Vrang N (2009) The effect of leptin receptor deficiency and fasting on cannabinoid receptor 1 mRNA expression in the rat hypothalamus, brainstem and nodose ganglion. Neurosci Lett 463(2):125–129
Johnstone LE, Fong TM, Leng G (2006) Neuronal activation in the hypothalamus and brainstem during feeding in rats. Cell Metab 4(4):313–321
Kamatchi GL, Rathanaswami P (2012) Inhibition of deprivation-induced food intake by GABAA antagonists: roles of the hypothalamic, endocrine and alimentary mechanisms. J Clin Biochem Nutr 51:19–26
Kangas BD, Delatte MS, Vemuri VK, Thakur GA, Nikas SP, Subramanian KV, Shukla VG, Makriyannis A, Bergman J (2013) Cannabinoid discrimination and antagonism by CB1 neutral and inverse agonist antagonists. J Pharmacol Exp Ther 344(3):561–567
Kelley AE, Baldo BA, Pratt WE (2005) A proposed hypothalamic–thalamic–striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol 493(1):72–85
Kim KW, Zhao L, Parker KL (2009) Central nervous system-specific knockout of steroidogenic factor 1. Mol Cell Endocrinol 300(1–2):132–136
Kinyua AW, Yang DJ, Chang I, Kim KW (2016) Steroidogenic factor 1 in the ventromedial nucleus of the hypothalamus regulates age-dependent obesity. PLoS One 11(9):e0162352
Kirkham TC, Williams CM, Fezza F, Marzo VD (2002) Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br J Pharmacol 136(4):550–557
Klöckener T, Hess S, Belgardt BF, Paeger L, Verhagen LA, Husch A, Sohn JW, Hampel B, Dhillon H, Zigman JM, Lowell BB (2011) High-fat feeding promotes obesity via insulin receptor/PI3K-dependent inhibition of SF-1 VMH neurons. Nat Neurosci 14(7):911
Kumarnsit E, Johnstone LE, Leng G (2003) Actions of neuropeptide Y and growth hormone secretagogues in the arcuate nucleus and ventromedial hypothalamic nucleus. Eur J Neurosci 17(5):937–944
Kuperman Y, Chen A (2008) Urocortins: emerging metabolic and energy homeostasis perspectives. Trends Endrocrinol Metab 19(4):122–129
Lee SH, Zabolotny JM, Huang H, Lee H, Kim YB (2016) Insulin in the nervous system and the mind: functions in metabolism, memory, and mood. Mol Metab 5(8):589–601
Li SB, de Lecea L (2020) The hypocretin (orexin) system: from a neural circuitry perspective. Neuropharmacology 167:107993
Li J, Hu Z, de Lecea L (2014) The hypocretins/orexins: integrators of multiple physiological functions. Br J Pharmacol 171(2):332–350
López M, Lage R, Saha AK, Pérez-Tilve D, Vázquez MJ, Varela L, Sangiao-Alvarellos S, Tovar S, Raghay K, Rodríguez-Cuenca S, Deoliveira RM (2008) Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell Metab 7(5):389–399
Lovejoy DA, Chang BS, Lovejoy NR, del Castillo J (2014) Molecular evolution of GPCRs: CRH/CRH receptors. J Mol Endocrinol 52(3):43–60
Lu XY, Bagnol D, Burke S, Akil H, Watson SJ (2000) Differential distribution and regulation of OX1 and OX2 orexin/hypocretin receptor messenger RNA in the brain upon fasting. Horm Behav 37(4):335–344
Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M (2001) Elmquist JK (2001) Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol 435(1):6–25
Martynska L, Wolinska-Witort E, Chmielowska M, Bik W, Baranowska B (2005) The physiological role of orexins. Neuro Endocrinol Lett 26(4):289–292
Mason BL, Wang Q, Zigman JM (2014) The central nervous system sites mediating the orexigenic actions of ghrelin. Annu Rev 76:519–533
Matias I, Di Marzo V (2007) Endocannabinoids and the control of energy balance. Trends Endrocrinol Metab 18(1):27–37
Meguid MM, Fetissov SO, Varma M, Sato T, Zhang L, Laviano A, Rossi-Fanelli F (2000) Hypothalamic dopamine and serotonin in the regulation of food intake. Nutrition 16(10):843–857
Merkestein M, Van Gestel MA, Van Der Zwaal EM, Brans MA, Luijendijk MC, Van Rozen AJ, Hendriks J, Garner KM, Boender AJ, Pandit R, Adan R (2014) GHS-R1a signaling in the DMH and VMH contributes to food anticipatory activity. Int J Obes 38(4):610–618
Messina A, Monda V, Villano I, Valenzano AA, Salerno M, Tafuri D, Avola R, Chieffi S, Sullo A, Cibelli G, Monda M (2017) Orexin system increases energy expenditure by brown adipose tissue activity. Natl J Physiol Pharm Pharmacol 7(7):658
Milbank E, López M (2019) Orexins/hypocretins: key regulators of energy homeostasis. Front Endocrinol 10:830
Millington GW (2007) The role of proopiomelanocortin (POMC) neurones in feeding behaviour. Nutr Metab 4(1):18
Mitchell SE, Nogueiras R, Morris A, Tovar S, Grant C, Cruickshank M, Rayner DV, Dieguez C, Williams LM (2009) Leptin receptor gene expression and number in the brain are regulated by leptin level and nutritional status. J Physiol 587(14):3573–3585
Morris DL, Rui L (2009) Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab 297(6):1247–1259
Myers MG Jr, Münzberg H, Leinninger GM, Leshan RL (2009) The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metab 9(2):117–123
Noble EE, Billington CJ, Kotz CM, Wang C (2011) The lighter side of BDNF. Am J Physiol-Reg I 300(5):1053–1069
Olszewski PK, Levine AS (2004) characterization of influence of central nociceptin/orphanin FQ on consummatory behavior. Endocrinology 145(6):2627–2632
Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R (2006) The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev 27(1):73–100
Pang ZP, Han W (2012) Regulation of synaptic functions in central nervous system by endocrine hormones and the maintenance of energy homoeostasis. Biosci Rep 32(5):423–432
Parker JA, Bloom SR (2012) Hypothalamic neuropeptides and the regulation of appetite. Neuropharmacology 63(1):18–30
Passani MB, Blandina P, Torrealba F (2011) The histamine H3 receptor and eating behavior. J Pharmacol Exp Ther 336(1):24–29
Pelleymounter MA, Joppa M, Carmouche M, Cullen MJ, Brown B, Murphy B, Grigoriadis DE, Ling N, Foster AC (2000) Role of corticotropin-releasing factor (CRF) receptors in the anorexic syndrome induced by CRF. J Pharmacol Exp Ther 293(3):799–806
Rafiei M, Taati M, Alavi S, Nayebzadeh H, Zendehdel M (2011) Effects of intracerebroventricular injection of histamine and H1, H2 receptor antagonists on electrocardiographic parameters in broiler chickens. Iran J Vet Res 12(3):192–198
Ramos-Lobo AM, Donato J Jr (2017) The role of leptin in health and disease. Temperature 4(3):258–291
Sainsbury A, Shi YC, Zhang L, Aljanova A, Lin Z, Nguyen AD, Herzog H, Lin S (2010) Y4 receptors and pancreatic polypeptide regulate food intake via hypothalamic orexin and brain-derived neurotropic factor dependent pathways. Neuropeptides 44(3):261–268
Saito ES, Kaiya H, Tachibana T, Tomonaga S, Denbow DM, Kangawa K, Furuse M (2005) Inhibitory effect of ghrelin on food intake is mediated by the corticotropin-releasing factor system in neonatal chicks. Regul Pept 125(1–3):201–208
Sakata T, Yoshimatsu H, Masaki T, Tsuda K (2003) Anti-obesity actions of mastication driven by histamine neurons in rats. Exp Biol Med 228(10):1106–1110
Sánchez-Lasheras C, Könner AC, Brüning JC (2010) Integrative neurobiology of energy homeostasis-neurocircuits, signals and mediators. Front Neuroendocrin 31(1):4–15
Segal JP, Stallings NR, Lee CE, Zhao L, Socci N, Viale A, Harris TM, Soares MB, Childs G, Elmquist JK, Parker KL (2005) Use of laser-capture microdissection for the identification of marker genes for the ventromedial hypothalamic nucleus. J Neurosci 25(16):4181–4188
Sekino A, Ohata H, Mano-Otagiri A, Arai K, Shibasaki T (2004) Both corticotropin-releasing factor receptor type 1 and type 2 are involved in stress-induced inhibition of food intake in rats. Psychopharmacology 176(1):30–38
Sherwin RS (2008) Bringing light to the dark side of insulin: a journey across the blood-brain barrier. Diabetes 57(9):2259–2268
Shimazu T, Minokoshi Y (2017) Systemic glucoregulation by glucose-sensing neurons in the ventromedial hypothalamic nucleus (VMH). J Clin Endocrinol Metab 1(5):449–459
Shiuchi T, Haque MS, Okamoto S, Inoue T, Kageyama H, Lee S, Toda C, Suzuki A, Bachman ES, Kim YB, Sakurai T (2009) Hypothalamic orexin stimulates feeding-associated glucose utilization in skeletal muscle via sympathetic nervous system. Cell Metab 10(6):466–480
Siljee JE, Unmehopa UA, Kalsbeek A, Swaab DF, Fliers E, Alkemade A (2013) Melanocortin 4 receptor distribution in the human hypothalamus. Eur J Endocrinol 168(3):361–369
Simon V, Cota D (2017) Mechanisms in endocrinology: endocannabinoids and metabolism: past, present and future. Eur J Endocrinol 176(6):309–324
Smith ML, Prall B, Nandar W, Cline MA (2008) β-melanocyte-stimulating hormone potently reduces appetite via the hypothalamus in chicks. J Neuroendocrinol 20(2):220–226
Sohn JW, Oh Y, Kim KW, Lee S, Williams KW, Elmquist JK (2016) Leptin and insulin engage specific PI3K subunits in hypothalamic SF1 neurons. Mol Metab 5(8):669–679
Stanley BG, Urstadt KR, Charles JR, Kee T (2011) Glutamate and GABA in lateral hypothalamic mechanisms controlling food intake. Physiol Behav 104(1):40–46
Stengel A, Taché YF (2014) CRF and urocortin peptides as modulators of energy balance and feeding behavior during stress. Front Neurosci 8:52–62
Sternson SM, Shepherd GM, Friedman JM (2005) Topographic mapping of VMH → arcuate nucleus microcircuits and their reorganization by fasting. Nat Neurosci 8(10):1356–1363
Suyama S, Yada T (2018) New insight into GABAergic neurons in the hypothalamic feeding regulation. J Physiol Sci 68(6):717–722
Tabarean IV (2016) Histamine receptor signaling in energy homeostasis. Neuropharmacology 106:13–19
Takano S, Kanai S, Hosoya H, Ohta M, Uematsu H, Miyasaka K (2004) Orexin-A does not stimulate food intake in old rats. Am J Physiol Gastrointest Liver Physiol 287(6):1182–1187
Tibiriça E (2010) The multiple functions of the endocannabinoid system: a focus on the regulation of food intake. Diabetol Metab Syndr 2(1):5
Timper K, Brüning JC (2017) Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. DMM 10(6):679–689
Toda C, Shiuchi T, Lee S, Yamato-Esaki M, Fujino Y, Suzuki A, Okamoto S, Minokoshi Y (2009) Distinct effects of leptin and a melanocortin receptor agonist injected into medial hypothalamic nuclei on glucose uptake in peripheral tissues. Diabetes 58(12):2757–2765
Tong Q, Ye C, McCrimmon RJ, Dhillon H, Choi B, Kramer MD, Yu J, Yang Z, Christiansen LM, Lee CE, Choi CS (2007) Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab 5(5):383–393
Torrealba F, Riveros ME, Contreras M, Valdes JL (2012) Histamine and motivation Front Syst Neurosci 6:51
Turenius CI, Htut MM, Prodon DA, Ebersole PL, Ngo PT, Lara RN, Wilczynski JL, Stanley BG (2009) GABAA receptors in the lateral hypothalamus as mediators of satiety and body weight regulation. Brain Res 1262:16–24
Vanevski F, Xu B (2013) Molecular and neural bases underlying roles of BDNF in the control of body weight. Front Neurosci 7:37
Vergoni AV, Bertolini A (2000) Role of melanocortins in the central control of feeding. Eur J Pharmacol 405(1–3):25–32
Vogt MC, Brüning JC (2013) CNS insulin signaling in the control of energy homeostasis and glucose metabolism–from embryo to old age. Trends Endrocrinol Metab 24(2):76–84
Vucetic Z, Reyes TM (2010) Central dopaminergic circuitry controlling food intake and reward: implications for the regulation of obesity. Wires Syst Biol Med 2(5):577–593
Wallén-Mackenzie Å, Wootz H, Englund H (2010) Genetic inactivation of the vesicular glutamate transporter 2 (VGLUT2) in the mouse: what have we learnt about functional glutamatergic neurotransmission? Ups J Med Sci 115(1):11–20.
Wang W, Andersson M, Iresjö BM, Lönnroth C, Lundholm K (2006) Effects of ghrelin on anorexia in tumor-bearing mice with eicosanoid-related cachexia. Int J Oncol 28(6):1393–1400
Wang L, Stengel A, Goebel M, Martinez V, Gourcerol G, Rivier J, Taché Y (2011) Peripheral activation of corticotropin-releasing factor receptor 2 inhibits food intake and alters meal structures in mice. Peptides 32(1):51–59
Wang S, Khondowe P, Chen S, Yu J, Shu G, Zhu X, Wang L, Gao P, Xi Q, Zhang Y, Jiang Q (2012) Effects of" Bioactive" amino acids leucine, glutamate, arginine and tryptophan on feed intake and mRNA expression of relative neuropeptides in broiler chicks. J Anim Sci Biotechnol 3(1):27
Waterson MJ, Horvath TL (2015) Neuronal regulation of energy homeostasis: beyond the hypothalamus and feeding. Cell metab 22(6):962–970
Williams G, Cai XJ, Elliott JC, Harrold JA (2004) Anabolic neuropeptides. Physiol Behav 81(2):211–222
Woods SC, D'Alessio DA (2008) Central control of body weight and appetite. J Clin Endocrinol Metab 93:37–50
Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, Tecott LH, Reichardt LF (2003) Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci 6(7):736–742
Yanagi S, Sato T, Kangawa K, Nakazato M (2018) The homeostatic force of ghrelin. Cell metab 27(4):786–804
Yoshimatsu H (2006) The neuronal histamine H1 and pro-opiomelanocortin–melanocortin 4 receptors: independent regulation of food intake and energy expenditure. Peptides 27(2):326–332
Yousefvand S, Hamidi F (2019) Role of paraventricular nucleus in regulation of feeding behaviour and the design of intranuclear neuronal pathway communications. Int J Pept Res Ther 2019:1–2
Yousefvand S, Hamidi F, Zendehdel M, Parham A (2018) Effects of insulin and somatostatin on water intake in neonatal chickens. I J Physiol Pharmacol 2(3):165–158
Yousefvand S, Hamidi F, Zendehdel M, Parham A (2019) Interaction of neuropeptide Y receptors (NPY1, NPY2 and NPY5) with somatostatin on somatostatin-induced feeding behaviour in neonatal chicken. Br Poult Sci 60(1):71–78
Yousefvand S, Hamidi F, Zendehdel M, Parham A (2020) Survey the effect of insulin on modulating feed intake via NPY receptors in 5-day-old chickens. Int J Pept Res Ther 26(1):467–476
Zendehdel M, Hassanpour S (2014) Central regulation of food intake in mammals and birds: a review. Neurotransmitter 1:1–7
Zendehdel M, Mokhtarpouriani K, Babapour V, Pourrahimi M, Hamidi F (2013a) The role of 5-HT2A and 5-HT2C receptors on harmalineinduced eating behavior in 24-h food-deprived broiler cockerels. Iran J Vet Res 14(2):94–99
Zendehdel M, Mokhtarpouriani K, Hamidi F, Montazeri R (2013b) Intracerebroventricular injection of ghrelin produces hypophagia through central serotonergic mechanisms in chicken. Vet Res Commun 37(1):37–41
Zendehdel M, Hamidi F, Hassanpour S (2015) The effect of histaminergic system on nociceptin/orphanin FQ induced food intake in chicken. Int J Pept Res Ther 21(2):179–186
Zendehdel M, Parvizi Z, Hassanpour S, Baghbanzadeh A, Hamidi F (2017) Interaction between nociceptin/orphanin FQ and adrenergic system on food intake in neonatal chicken. Int J Pept Res Ther 23(1):155–161
Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK (2006) Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol 494(3):528–548
Acknowledgements
The authors would like to thank you to Ferdowsi University of Mashhad, for their support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have not conflicts of interest.
Research Involving Human Participants and/or Animals
The authors declare that there is no conflict of interest regarding the publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yousefvand, S., Hamidi, F. The Role of Ventromedial Hypothalamus Receptors in the Central Regulation of Food Intake. Int J Pept Res Ther 27, 689–702 (2021). https://doi.org/10.1007/s10989-020-10120-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-020-10120-9