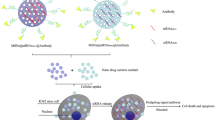
Abstract
Glucocorticoid, such as dexamethasone (Dex) is often used along with chemotherapy to antagonize side effects of chemotherapy. However, sustained use of Dex frequently develops drug resistance in patients. As a strategy to re-induce drug sensitivity, we planned to modify Dex by chemically conjugating it with twin ten carbon aliphatic chain containing cationic lipid. The resultant molecule, DX10, inhibited STAT3 activation through lowering the production of IL-6. To enhance the STAT3 inhibitory effect of DX10, we used WP (a commercially available STAT3 inhibitor) along with DX10. Combination treatment of both significantly inhibited STAT3 activation when compared to either of the individual treatment. The effect of DX10, either in combination or alone, was mediated through glucocorticoid receptor (GR), thereby repurposing the role of GR in the context of p-STAT3 inhibition-mediated cancer treatment. Cellular viability study proved the synergistic effect of WP and DX10. Further, combination treatment led to induction of early stage of apoptosis and cell cycle arrest. In vivo melanoma tumor regression study confirmed the enhanced anti-tumor activity of co-treatment over individual treatment of DX10 or WP. Thus, together our result demonstrates that DX10 may be used in combination therapy with STAT3 inhibitor like WP for combating cancer with constitutively active STAT3.
Graphical abstract












Similar content being viewed by others
References
Gottesman MM (2002) Mechanisms of cancer drug resistance. Annu Rev Med 53:615–627
Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Kinzler KW (2013) Cancer genome landscapes. Science 339:1546–1558
Housman G, Byler S, Heerboth S, Lapinska K, Longacre M, Snyder N, Sarkar S (2014) Drug resistance in cancer: an overview. Cancers 6:1769–1792
O’Shaughnessy J, Osborne C, Pippen JE, Yoffe M, Patt D, Rocha C, Koo IC, Sherman BM, Bradley C (2011) Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med 364:205–214
Amadori D, Cecconetto L (2006) Gemcitabine and taxanes in metastatic breast cancer. Ann Oncol 17:173–176
Rocha LC, Sherman CA, Brescia FJ, Brunson CY, Green MR (2001) Irinotecan/gemcitabine combination chemotherapy in pancreatic cancer. Oncology 15:46–51
Akira S (1999) Functional roles of STAT family proteins: lessons from knockout mice. Stem Cells 17:138–146
Garcia R, Bowman TL, Niu G, Yu H, Minton S, Muro-Cacho CA et al (2001) Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene 20:2499–2513
Gouilleux-Gruart V, Gouilleux F, Desaint C, Claisse JF, Capiod JC, Delobel J et al (1996) STAT-related transcription factors are constitutively activated in peripheral blood cells from acute leukemia patients. Blood 87:1692–1697
Fernandes A, Hamburger AW, Gerwin BI (1999) ErbB-2 kinase is required for constitutive stat 3 activation in malignant human lung epithelial cells. Int J Cancer 83:564–570
Simonian PL, Grillot DA, Nuñez G (1997) Bcl-2 and Bcl-XL can differentially block chemotherapy-induced cell death. Blood 90:1208–1216
Kunigal S, Lakka SS, Sodadasu PK, Estes N, Rao JS (2009) Stat3-siRNA induces Fas-mediated apoptosis in vitro and in vivo in breast cancer. Int J Oncol 34:1209–1220
Alas S, Bonavida B (2003) Inhibition of constitutive STAT3 activity sensitizes resistant non-Hodgkin’s lymphoma and multiple myeloma to chemotherapeutic drug-mediated apoptosis. Clin Cancer Res 9:316–326
Herr I, Ucur E, Herzer K, Okouoyo S, Ridder R, Krammer PH et al (2003) Glucocorticoid cotreatment induces apoptosis resistance toward cancer therapy in carcinomas. Cancer Res 63:3112–3120
Rutz HP (2002) Effects of corticosteroid use on treatment of solid tumours. Lancet 360:1969–1970
Rutz HP, Herr I (2004) Interference of glucocorticoids with apoptosis signaling and host-tumor interactions. Cancer Biol Ther 3:715–718
Kriegler AB, Bernardo D, Verschoor SM (1994) Protection of murine bone marrow by dexamethasone during cytotoxic chemotherapy. Blood 83:65–71
Harousseau JL, Attal M, Leleu X, Troncy J, Pegourie B, Stoppa AM et al (2006) Bortezomib plus dexamethasone as induction treatment prior to autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: results of an IFM phase II study. Haematologica 91:1498–1505
Sui M, Chen F, Chen Z, Fan W (2006) Glucocorticoids interfere with therapeutic efficacy of paclitaxel against human breast and ovarian xenograft tumors. Int J Cancer 119:712–717
Sau S, Banerjee R (2014) Cationic lipid-conjugated dexamethasone as a selective antitumor agent. Eur J Med Chem 83:433–447
Ferrajoli A, Faderl S, Van Q, Koch P, Harris D, Liu Z et al (2007) WP1066 disrupts Janus kinase-2 and induces caspase-dependent apoptosis in acute myelogenous leukemia cells. Cancer Res 67:11291–11299
Iwamaru A, Szymanski S, Iwado E, Aoki H, Yokoyama T, Fokt I et al (2007) A novel inhibitor of the STAT3 pathway induces apoptosis in malignant glioma cells both in vitro and in vivo. Oncogene 26:2435–2444
Tallarida RJ (2001) Drug synergism: its detection and applications. J Pharmacol Exp Ther 298:865–872
Chou TC (2010) Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res 70:440–446
Kong LY, Abou-Ghazal MK, Wei J, Chakraborty A, Sun W, Qiao W et al (2008) A novel inhibitor of signal transducers and activators of transcription 3 activation is efficacious against established central nervous system melanoma and inhibits regulatory T cells. Clin Cancer Res 14:5759–5768
Takeda T, Kurachi H, Yamamoto T, Nishio Y, Nakatsuji Y, Morishige KI et al (1998) Crosstalk between the interleukin-6 (IL-6)-JAK-STAT and the glucocorticoid-nuclear receptor pathway: synergistic activation of IL-6 response element by IL-6 and glucocorticoid. J Endocrinol 159:323–330
Zhang Z, Jones S, Hagood JS, Fuentes NL, Fuller GM (1997) STAT3 acts as a co-activator of glucocorticoid receptor signaling. J Biol Chem 272:30607–30610
Verhoog NJ, Du Toit A, Avenant C, Hapgood JP (2011) Glucocorticoid-independent repression of tumor necrosis factor (TNF) alpha-stimulated interleukin (IL)-6 expression by the glucocorticoid receptor: a potential mechanism for protection against an excessive inflammatory response. J Biol Chem 286:19297–19310
Nishimura K, Nonomura N, Satoh E, Harada Y, Nakayama M, Tokizane T et al (2001) Potential mechanism for the effects of dexamethasone on growth of androgen-independent prostate cancer. J Natl Cancer Inst 93:1739–1746
Gross A, McDonnell JM, Korsmeyer SJ (1999) BCL-2 family members and the mitochondria in apoptosis. Genes Dev 13:1899–1911
Gottlieb TM, Leal JF, Seger R, Taya Y, Oren M (2002) Cross-talk between Akt, p53 and Mdm2: possible implications for the regulation of apoptosis. Oncogene 21:1299–1303
Nishimura K, Nonomura N, Satoh E, Harada Y, Nakayama M, Tokizane T et al (2001) Potential mechanism for the effects of dexamethasone on growth of androgen-independent prostate cancer. J Natl Cancer Inst 93:1739–1746
Waage A, Slupphaug G, Shalaby R (1990) Glucocorticoids inhibit the production of IL 6 from monocytes, endothelial cells and fibroblasts. Eur J Immunol 20:2439–2443
Obrador E, Valles SL, Benlloch M, Sirerol JA, Pellicer JA, Alcácer J, Coronado JAF, Estrela JM (2014) Glucocorticoid receptor knockdown decreases the antioxidant protection of B16 melanoma cells: an endocrine system-related mechanism that compromises metastatic cell resistance to vascular endothelium- induced tumor cytotoxicity. PLoS ONE 9(5):e96466
Acknowledgements
SS and SKM thank Council of Scientific and Industrial Research (CSIR), Government of India, New Delhi for their doctoral research fellowships. RB acknowledges financial assistance from CSIR Network Project Grant [CSC0302, BSC0123], Govt. of India.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Samaresh Sau and Sujan Kumar Mondal have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sau, S., Mondal, S.K., Kashaw, S.K. et al. Combination of cationic dexamethasone derivative and STAT3 inhibitor (WP1066) for aggressive melanoma: a strategy for repurposing a phase I clinical trial drug. Mol Cell Biochem 436, 119–136 (2017). https://doi.org/10.1007/s11010-017-3084-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-017-3084-z