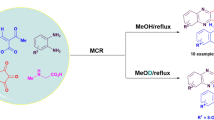
Abstract
An expedient route toward the synthesis of 4-hydroxyquinolone grafted spiropyrrolidines or pyrrolizidines has been accomplished through 1,3-dipolar cycloaddition reaction of various azomethine ylides derived from isatin or acenaphthalene and sarcosine with 4-hydroxyquinolone derivatives as dipolarophile. The regio and stereo chemical outcome of the cycloaddition reaction is ascertained by X-ray crystallographic studies and spectroscopic techniques of the cycloadducts. Furthermore, cytotoxicity evaluation of selected compounds showed significant inhibition of cell proliferation against cervical as well as colon cancer cell lines.










Similar content being viewed by others
References
Padwa A, Pearson WH (2003) Synthetic applications of 1, 3-dipolar cycloaddition chemistry toward heterocycles and natural products. 59. doi:10.1002/0471221902
Sarotti AM, Spanevello RA, Suarez AG, Echeverría GA, Piro OE (2012) 1,3-Dipolar cycloaddition reactions of azomethine ylides with a cellulose-derived chiral enone. A novelroute for organocatalysts development. Org Lett 14:2556–2559. doi:10.1021/ol3008588
Uma Maheswari S, Balamurugan K, Perumal S, Yogeeswari P, Sriram D (2010) A facile 1,3-dipolar cycloaddition of azomethine ylides to 2-arylidene-1,3-indanediones: synthesis of dispiro-oxindolylpyrrolothiazoles and their antimycobacterial evaluation. Bioorg Med Chem Lett 20:7278–7282. doi:10.1016/j.bmcl.2010.10.080
Yavuz S, Ozkan H, Tok G, Disli A (2013) Facile method for 1,3-dipolar cycloaddition reaction of azomethine ylides: highly stereoselective synthesis of substituted pyrrolidine derivatives. J Heterocycl Chem 16:1437–1440. doi:10.1002/jhet.1540
Alcaide B, Almendros P, Alonso J M, Aly M F (2000) 1,3-Dipolar cycloaddition of 2- azetidinone-tethered azomethine ylides. Application to the rapid, stereo controlled synthesis of optically pure highly functionalized pyrrolizidine systems. Chem Comm 485–486. doi:10.1039/B000249F
Domling A, Ugi I (2000) Multicomponent reactions with isocyanides. Angew Chem Int Ed 39:3168–3210. doi:10.1002/1521-3773(20000915)39
Balme G, Bossharth E, Monteiro N (2003) Pd-assisted multicomponent synthesis of heterocycles. Eur J Org Chem 4101–4111: doi:10.1002/ejoc.200300378
Sridhar G, Gunasundari T, Raghunathan R (2007) A greener approach for the synthesis of 1-\(N\)-methyl-(spiro[2.3’]oxindol espiro[3.2”]spiro[2.3’]indan-1,3-dionespiro [2.2”]) cyclopentan one-4-aryl pyrrolidines. Tetrahedron Lett 48:319–322. doi:10.1016/j.tetlet.2006.11.002
Suresh Kumar R, Osman H, Perumal S, Menendez JC, Ashraf Ali M, Ismail R, Choon TS (2011) A facile three-component [3+2]-cycloaddition/annulation domino protocol for the regio- and diastereoselective synthesis of novel penta- and hexacyclic cage systems, involving the generation of two heterocyclic rings and five contiguous stereocenters. Tetrahedron 67:3132–3139. doi:10.1016/j.tet.2011.02.058
Jiang T, Kuhen KL, Wolff K, Yin H, Bieza K, Caldwell J, Bursulaya B, Wu T, He Y (2006) Design, synthesis and biological evaluations of novel oxindoles as HIV-1non- nucleoside reverse transcriptase inhibitors. Part I Bioorg Med Chem Lett 16:2105–2108. doi:10.1016/j.bmcl.2006.01.073
Dalpozzo R, Bartoli G, Bencivenni G (2012) Recent advances in organocatalytic methods for the synthesis of disubstituted 2- and 3-indolinones. Chem Soc Rev 41:7247–7290. doi:10.1039/C2CS35100E
Sebahar PR, Williams RM (2000) The asymmetric total synthesis of (+)- and (-)-spirotryprostatin B. J Am Chem Soc 122:5666–6667. doi:10.1021/ja001133n
Kang K, Matsumoto Y, Murakami H, Takayama M, Kitajima N, Watanabe H (2002) Pteropodine and isopteropodine positively modulate the function of rat muscarinic M(1) and 5-HT(2) receptors expressed in Xenopus oocyte. Eur J Pharmacol 444:39–45. doi:10.1016/S0014-2999(02)01608-4
Ma J, Hecht SM, Javaniside A (2004) Novel DNA cleavage agent from Alangium javanicum having an unusual oxindole skeleton. Chem Commun 10:1190–1191. doi:10.1039/B402925A
Usui T, Kondoh M, Cui CB, Mayumi T, Osada H, Tryprostatin A (1998) A specific and novel inhibitor of microtubule assembly. Biochem J 333:543–548
Abou-Gharbia MA, Doukas PH (1979) Synthesis of tricyclic arylspiro compounds as potential antileukemic and anticonvulsant agents. Heterocycles 12:637–640. doi:10.3987/R-1979-05-0637
Kornet MJ, Thio AP (1976) Oxindole-3-spiropyrrolidines and piperidines. Synthesis and local anesthetic activity. J Med Chem 19:892–898. doi:10.1021/jm00229a007
Lu Y, Nikolovska-Coleska Z, Fang X, Gao W, Shangary S, Qiu S, Qin D, Wang S (2006) Discovery of a nanomolar inhibitor of the human murine double minute 2 (MDM2)-p53 interaction through an integrated, virtual database screening strategy. J Med Chem 49:3759–3762. doi:10.1021/jm060023+
Ding K, Lu Y, Nikolovska-Coleska Z, Wang G, Qiu S, Shangary S, Gao W, Qin D, Stuckey J, Krajewski K, Roller PP, Wang S (2006) Structure-based design of spiro-oxindoles as potent, specific small-molecule inhibitors of the MDM2-p53 interaction. J Med Chem 49:3432–3435. doi:10.1021/jm051122a
Trost BM, Brennan MK (2009) Asymmetric syntheses of oxindole and indole spirocyclic alkaloid natural products. Synthesis 3003–3025: doi:10.1055/s-0029-1216975
Albertshofer K, Tan B, Barbas CF (2012) Assembly of spirooxindole derivatives containing four consecutive stereocenters via organocatalytic Michael-Henry cascade reactions. Org Lett 14:1834–1837. doi:10.1021/ol300441z
Chandraprakash K, Sankaran M, Uvarani C, Shankar R, Ata A, Dallemer F, Mohan PS (2013) A strategic approach to the synthesis of novel class of dispiroheterocyclic derivatives through 1,3 dipolar cycloaddition of azomethine ylide with (E)-3-arylidene-2,3-dihydro-8-nitro-4-quinolone. Tetrahedron Lett 54:3896. doi:10.1016/j.tetlet.2013.05.077
Castellano RK, Diederich F, Meyer EA (2003) Interactions with aromatic rings in chemical and biological recognition. Angew Chem Int Ed 42:1210–1250. doi:10.1002/anie.200390319
Waters ML (2002) Aromatic interactions in model systems. Current Opin in Chem Biol 6:736–741. doi:10.1016/S1367-5931(02)00359-9
Kollman PA (1977) Noncovalent interaction. Chem Rev 10:365–371. doi:10.1021/ar50118a003
Johnson ER, Keinan S, Mori-Sanchez P, Contreras-Garcia J, Cohen AJ, Yang W (2010) Revealing noncovalent interactions. J Am Chem Soc 132:6498–6506. doi:10.1021/ja100936w
Lu Y, Wang Y, Zhu W (2010) Nonbonding interactions of organic halogens in biological systems: implications for drug discovery and biomolecular design. Phys Chem Chem Phys 12:4543–4551. doi:10.1039/b926326h
Sotriffer C, Klebe G (2002) Identification and mapping of small molecule binding sites in proteins: computational tools for structure-based drug design. Il Farmaco 57:243–251. doi:10.1016/S0014-827X(02)01211-9
Munawar MA, Azad M, Athar M, Groundwater PW (2008) Synthesis and antimicrobial activity of quinoline-based 2-pyrazolines. Chem Pap 62:288–293. doi:10.2478/s11696-008-0025-z
Lakshmi NV, Thirumurugan P, Perumal PT (2010) An expedient approach for the synthesis of dispiropyrrolidine bisoxindoles, spiropyrrolidine oxindoles and spiroindane-1, 3-diones through 1,3-dipolar cycloaddition reactions. Tetrahedron Lett 51:1064–1068. doi:10.1016/j.tetlet.2009.12.079
Revathi K, Sankaran M, Ramesh P, Mohan PS, Ponnuswamy MN (2010) 4-Hydroxy-3-(10-methyl-2-oxo-40-phenylspiro[indoline-3,20-pyrrolidine]-30-ylcarbonyl) quinolin-(1H)- one. Acta Cryst E 66:o952. doi:10.1107/S1600536810010500
Vennila KN, Sankaran M, Mohan PS, Velmurugan D (2011) (1R*, 30S*, 40R*)-40-(4-Chlorophenyl)-30-[(4-hydroxy-2-oxo-1,2-di hydroquinolin-3-yl)carbonyl]-10-methylspiro- [acenaphthylene-1,20-pyrrolidin]-2-one. Acta Cryst E 67:o3376. doi:10.1107/S1600536811048896
Brazier JB, Evans G, Gibbs TJK, Coles SJ, Hursthouse MB, Platts JA, Tomkinson NCO (2008) Solution phase, solid state, and theoretical investigations on the MacMillan imidazolidinone. Org Lett 11:133–136. doi:10.1021/ol802512y
Biegler-König F, Schönbohm J, Bayles D (2001) AIM2000-A program to analyze and visualize atoms in molecules. J Comput Chem 22:545–559. doi:10.1002/1096-987X(20010415)22
Hansen C H, Brunner N (1994) Cell biology, Part I. Academic Press, New York, pp 16–18
TURBOMOLE V5.9 (2008) A development of University of Karlsruhe and Forschungszentrum Karlsruhe GmbH, 1989–2007, TURBOMOLE GmbH, since 2007; available from http://www.turbomole.com
Acknowledgments
The Authors thank the Council of Scientific and Industrial Research CSIR-SRF (Ref No. 09/472(0145)/2K10-EMR-I) New Delhi, India, for financial assistance. We acknowledge Dr. Aathirajan, Professor, Cell culture Laboratory, KMCH College of Pharmacy, Coimbatore for evaluating cytotoxicity of synthesized compounds for Cervical cancer cell lines. Dr. Karvembu, Head of the Department of Chemistry, National Institute of Technology, Trichy is gratefully acknowledged for providing optical rotatory dispersion studies. The authors thank SAIF, NMR Research Centre, Bangalore SAIF, IIT Chennai for spectral study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sankaran, M., Uvarani, C., Chandraprakash, K. et al. A regioselective multicomponent protocol for the synthesis of novel bioactive 4-hydroxyquinolin-2(1H)-one grafted monospiropyrrolidine and thiapyrrolizidine hybrids. Mol Divers 18, 269–283 (2014). https://doi.org/10.1007/s11030-013-9498-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-013-9498-y