Abstract
The synthesis of bis(1,2,4-oxadiazoles), 1,2,4-oxadiazolyl-quinazolines, and 1,2,4-oxadiazolyl-benzothiazinones has been investigated by the reaction of diaminoglyoxime with various ketones and methyl 2-aminobenzoate, 2-amino-5-chlorophenyl)(phenyl)methanone, and 2-mercapto benzoic acid in acetic acid either a catalyst or solvent at 100 \(^{\circ }\)C.
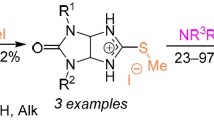
Graphical abstract










Similar content being viewed by others
References
Kakanejadifard A (2004) A modified-one pot synthesis of diaminoglyoxime. Iran J Chem Chem Eng 23:117–118. doi:1021-9986/04/1/117
Moghimi A, Hosseinzadeh Khanmiri R, Shaabani A, Hamadani H (2013) A green synthesis of nitrones from diamino glyoxime using aldehydes and ketones. J Iran Chem Soc 10:929–936. doi:10.1007/s13738-013-0230-8
Andrianov VG, Eremeev AV (1990) \(\sigma \)-Adducts in the 1,2,4-oxadiazole series. Chem Heterocycl Compd 26:714
Trusule M, Kupce E, Augustane I, Verovskii NV, Lukevics E, Baumane L, Gavars R, Stradins J (1991) Synthesis, antitumor activity, and electrochemical properties of furan-containing uracil derivatives. Khimiya Geterotsiklicheskikh Soedin 12:1687–1694
Moghimi A, Hosseinzadeh R, Omrani I, Shaabani A (2013) A new library of 4(3\(H)\)- and 4,4(3\(H\),3\(H)\)-quinazolinones and 2-(5-alkyl-1,2,4-oxadiazol-3-yl)quinazolin-4(3\(H)\)-one obtained from diaminoglyoxime. Tetrahedron Lett 54:3956–3959. doi: 10.1016/j.tetlet.2013.05.065
Carbone M, Li Y, Irace C, Mollo E, Castelluccio F, Pascale AD, Cimino G, Santamaria R, Guo YW, Gavagnin M (2011) Structure and cytotoxicity of phidianidines A and B: first finding of 1,2,4-oxadiazole system in a marine natural product. Org Lett 13:2516–2519. doi:10.1021/ol200234r
Ispikoudi M, Amvrazis M, Kontogiorgis C, Koumbis AE, Litinas KE, Hadjipavlou-Litina D, Fylaktakidou KC (2010) Convenient synthesis and biological profile of 5-amino-substituted 1,2,4-oxadiazole derivatives. Eur J Med Chem 45:5635–5645. doi:10.1016/j.ejmech.2010.09.016
Bokach NA, Khripoun AV, Kukushkin VY, Haukka M, Pombeiro AJL (2003) A route to 1,2,4-oxadiazoles and their complexes via platinum-mediated 1,3-dipolar cycloaddition of nitrile oxides to organonitriles. Inorg Chem 42:896–903. doi:10.1021/ic026103v
dos Santos Filho JM, Leite ACL, de Oliveira BG, Moreira DRM, Lima MS, Soares MBP, Leite LFCC (2009) Design, synthesis and cruzain docking of 3-(4-substituted-aryl)-1,2,4-oxadiazole-N-acylhydrazones as anti-Trypanosoma cruzi agents. Bioorg Med Chem 17:6682–6691. doi:10.1016/j.bmc.2009.07.068
Rai NP, Narayanaswamy VK, Govender T, Manuprasad BK, Shashikanth S, Arunachalam PN (2010) Design, synthesis characterization, and antibacterial activity of {5-chloro-2-[(3-substitutedphenyl-1,2,4-oxadiazol-5-yl)-methoxy]-phenyl}-(phenyl)-methanones. Eur J Med Chem 45:2677–2682. doi:10.1016/j.ejmech.2010.02.021
Masahiro O, Mamoru H, Hideo S, Morio N (2008) Development of novel beta-amyloid probes based on 3,5-diphenyl-1,2,4-oxadiazole. Bioorg Med Chem 17:6867–6672. doi:10.1016/j.bmc.2008.05.054
Srikantamurthy N, Umesha KB, Shubakara K, Chethan J (2013) Novel pyrazoline amidoxime and their 1,2,4-oxadiazole analogues: synthesis and pharmacological screening. Bioorg Med Chem Lett 23:4532–4539. doi:10.1016/j.bmcl.2013.06.042
Borg S, Luthman K, Nyberg F, Terenius L, Hacksel U (1993) 1,2,4-Oxadiazole derivatives of phenylalanine: potential inhibitors of substance P endopeptidase. Eur J Med Chem 28:801–806. doi:10.1016/0223-5234(93)90115-U
Malamas MS, Sredy J, McCaleb M, Gunawan I, Mihan B, Sullivan D (2001) Antihyperglycemic activity of new 1,2,4-oxadiazolidine-3,5-diones. Eur J Med Chem 36:31–42. doi:10.1016/S0223-5234(00)01191-0
Zhang HZ, Kasibhatla S, Kuemmerle J, Kemnitzer W, Ollis-Mason K, Qiu L, Crogan-Grundy C, Tseng B, Drewe J, Cai SX (2005) Discovery and structure-activity relationship of 3-aryl-5-aryl-1,2,4-oxadiazoles as a new series of apoptosis inducers and potential anticancer agents. J Med Chem 48:5215–5223. doi:10.1021/jm050292k
Kumar D, Patel G, Johnson EO, Shah K (2009) Synthesis and anticancer activities of novel 3,5-disubstituted-1,2,4-oxadiazoles. Bioorg Med Chem Lett 19:2739–2741. doi:10.1016/j.bmcl.2009.03.158
Kemnitzer W, Kuemmerle J, Zhang HZ, Kasibhatla S, Tseng B, Drewe J, Cai SX (2009) Discovery of 3-aryl-5-aryl-1,2,4-oxadiazoles as a new series of apoptosis inducers. 2. Identification of more aqueous soluble analogs as potential anticancer agents. Bioorg Med Chem Lett 19:4410–4415. doi:10.1016/j.bmcl.2009.05.052
Burns AR, Kerr JH, Kerr WJ, Passmore J, Paterson LC, Watson AJB (2010) Tuned methods for conjugate addition to a vinyl oxadiazole; synthesis of pharmaceutically important motifs. Org Biomol Chem 8:2777–2783. doi:10.1039/c001772h
Kiss LE, Ferreira HS, Torrao L, Bonifacio MJ, Palma PN, Soares-da-Silva P, Learmonth DA (2010) Discovery of a long-acting, peripherally selective inhibitor of catechol-O-methyltransferase. J Med Chem 53:3396–3411. doi:10.1021/jm1001524
Filho JMDS, Leite ACL, Oliveira BG, Moreira DRM, Lima MS, Soares MBP, Leite LFCC (2009) Design, synthesis and cruzain docking of 3-(4-substituted-aryl)-1,2,4-oxadiazole-N-acylhydrazones as anti-Trypanosoma cruzi agents. Bioorg Med Chem 17:6682–6691. doi:10.1016/j.bmc.2009.07.068
Terenzi A, Barone G, Piccionello AP, Giorgi G, Guarcello A, Portanova P, Calvaruso G, Buscemi S, Vivona N, Pace A (2010) Synthesis, characterization, cellular uptake and interaction with native DNA of a bis(pyridyl)-1,2,4-oxadiazole copper(II) complex. Dalton Trans 39:9140–9145. doi:10.1039/c0dt00266f
Piccionello AP, Musumeci R, Cocuzza C, Fortuna CG, Guarcello A, Pierro P, Pace A (2012) Synthesis and preliminary antibacterial evaluation of Linezolid-like 1,2,4-oxadiazole derivatives. Eur J Med Chem 50:441–448. doi:10.1016/j.ejmech.2012.02.002
Hugo G, Rodrigo C, André AV, Rajendra MS (2008) Sonogashira coupling applied in the synthesis of 1,2,4-oxadiazole-based nonsymmetrical liquid crystals. Synthesis 4:605–609 1055/s-2008-1032156
Pace A, Pierro P (2009) The new era of 1,2,4-oxadiazoles. Org Biomol Chem 7:4337–4348. doi:10.1039/b908937c
Terenzi A, Barone G, Piccionello AP, Giorgi G, Guarcello A, Pace A (2011) Synthesis and chemical characterization of CuII, NiII and ZnII complexes of 3,5-bis(2\({}^\prime \)-pyridyl)-1,2,4-oxadiazole and 3-(2\({}^\prime \)-pyridyl)5-(phenyl)-1,2,4-oxadiazole ligands. Inorg Chim Acta 373:62–67. doi: 10.1016/j.ica.2011.03.057
Chris R, Peter JS (2007) The first metal complexes of 3,3\(^{\prime } \)-bi-1,2,4-oxadiazole: a curiously ignored ligand. Inorg Chem Commun 10:884–887. doi: 10.1016/j.inoche.2007.04.020
Terenzi A, Barone G, Palumbo Piccionello A, Giorgi G, Guarcello A, Portanova P, Calvaruso G, Buscemi S, Vivona N, Pace A (2010) Synthesis, characterization, cellular uptake and interaction with native DNA of a bis(pyridyl)-1,2,4-oxadiazole copper(II) complex. Dalton Trans 39:9140–9145. doi:10.1039/c0dt00266f
Mazaahir K, Suchi K, Shewta R, Kavita S (2007) Microwave accelerated multicomponent synthesis for a novel scaffold of monastrol analogues. Lett Org Chem 4:357–361. doi:10.2174/157017807781212085
Maggio B, Daidone G, Raffa D, Plescia S, Mantione L, Cutuli V, Mangano NG, Caruso A (2001) Synthesis and pharmacological study of ethyl 1-methyl-5-(substituted 3,4-dihydro-4-oxoquinazolin-3-yl)-1\(H\)-pyrazole-4-acetates. Eur J Med Chem 36:737–742. doi: 10.1016/S0223-5234(01)01259-4
Grover G, Kini SG (2006) Synthesis and evaluation of new quinazolone derivatives of nalidixic acid as potential antibacterial and antifungal agents. Eur J Med Chem 41:256–262. doi:10.1016/j.ejmech.2005.09.002
Roopan SM, Maiyalagan T, Khan FN (2008) Solvent-free syntheses of some quinazolin-4(3H)-ones derivatives. Can J Chem 86:1019–1025. doi:10.1139/v08-149
Chandrika PM, Yakaiah T, Raghu RRA, Narsaiah B, Chakra RN, Sridhar V, Venkateshwara RJ (2008) Synthesis of novel 4,6-disubstituted quinazoline derivatives, their anti-inflammatory and anti-cancer activity (cytotoxic) against U937 leukemia cell lines. Eur J Med Chem 43:846–852. doi:10.1016/j.ejmech.2007.06.010
Alagarsamy V, Pathak US (2007) Synthesis and antihypertensive activity of novel 3-benzyl-2-substituted-3\(H\)-[1,2,4]triazolo[5,1-b]quinazolin-9-ones. Bioorg Med Chem 15:3457–3462. doi: 10.1016/j.bmc.2007.03.007
Corona LD, Signorelli G, Pinzetta A, Coppi G (1992) Synthesis and immune stimulating activity of new 1,4-benzothiazine derivatives. Eur J Med Chem 27:419–423. doi:10.1016/0223-5234(92)90157-V
Fujita M, Ito S, Ota A, Kato N, Yamamoto K, Kawashima Y, Yamauchi H, Iwao J (1990) Synthesis and Ca\(^{2+}\) antagonistic activity of 2-[2-[(aminoalkyl)oxy]-5-methoxyphenyl]-3,4-dihydro-4-methyl-3-oxo-2\(H\)- 1,4-benzothiazines. J Med Chem 33:1898–1905. doi: 10.1021/jm00169a010
Makarov V, Manina G et al (2009) Benzothiazinones kill Mycobacterium tuberculosis by blocking arabinan synthesis. Science 324:801. doi:10.1126/science.1171583
Trefzer C, Rengifo-Gonzalez M, Hinner MJ, Schneider P, Makarov V, Cole ST, Johnsson K (2010) Benzothiazinones: prodrugs that covalently modify the decaprenylphosphoryl-\(\beta \)-D-ribose 2’-epimerase DprE1 of Mycobacterium tuberculosis. J Am Chem Soc 132:13663–13665. doi: 10.1021/ja106357w
Ribeiro AL, Degiacomi G, Ewann F, Buroni S, Incandela ML, Chiarelli LR, Mori G, Kim J, Contreras-Dominguez M, Park YS, Han SJ, Brodin P, Valentini G, Rizzi M, Riccardi G, Pasca MR (2011) Analogous mechanisms of resistance to benzothiazinones and dinitrobenzamides in Mycobacterium smegmatis. PLoS One 6:e26675. doi:10.1371/journal.pone.0026675
Shaabani A, Rezayan A, Keshipour S, Sarvary A, Ng SW (2009) A novel one-pot three-(in situ five-) component condensation reaction: an unexpected approach for the synthesis of tetrahydro-2,4-dioxo-1\(H\)-benzo[b][1,5]diazepine-3-yl-2-methylpropanamide derivatives. Org Lett 11:3342–3345. doi: 10.1021/ol901196z
Shaabani A, Maleki A, Moghimi-Rad J (2007) A novel isocyanide-based three-component reaction: synthesis of highly substituted 1,6-dihydropyrazine-2,3-dicarbonitrile derivatives. J Org Chem 72:6309–6311. doi:10.1021/jo0707131
Shaabani A, Maleki A, Mofakham H (2008) A novel one-pot pseudo-five-component synthesis of 4,5,6,7-tetrahydro-1H-1,4-diazepine-5-carboxamide derivatives. J Org Chem 73:3925–3927. doi:10.1021/jo8002612
Coskun N (1997) Regio and diastereoselective addition of imidazoline 3-oxides to aryl isocyanates. Tetrahedron 53:13873–13882. doi:10.1016/S0040-4020(97)00876-4
Cao XQ, Li ZX, Zhong WX, Qiu LH, Chen GQ (2009) Convenient synthesis of (\(Z)\)-4-amino-5-(hydroxyimino)-2,5-dihydro-1\(H\)-imidazole 3-oxide. Heterocycles 78:1445. doi: 10.3987/COM-08-11569
Acknowledgments
We gratefully acknowledge financial support from the Research Council of Imam Hossein and Shahid Beheshti Universities. We also wish to thank the Ministry of Higher Education of Malaysia (Grant No. UM.C/HIR/MOHE/SC/12) for supporting this study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khanmiri, R.H., Moghimi, A., Shaabani, A. et al. Diaminoglyoxime as a versatile reagent in the synthesis of bis(1,2,4-oxadiazoles), 1,2,4-oxadiazolyl-quinazolines and 1,2,4-oxadiazolyl-benzothiazinones. Mol Divers 18, 769–776 (2014). https://doi.org/10.1007/s11030-014-9536-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-014-9536-4