Abstract
Spirooxindoles are important synthetic targets possessing extended biological activity and drug discovery applications. This review focuses on the various strategies for the enantioselective synthesis of spirocyclic oxindoles relying on reports over the past decade and from earlier work. The spirooxindoles in this review are separated into three structural classes, and then further categorized into the method type from which the spirocycle is generated.





































































































































Similar content being viewed by others
References
Lipson VV, Zamigajlo LL, Petrova ON (2011) Development of 11\({\beta }\)-HSD1 inhibitors for the treatment of metabolic syndrome. Ukrainica Bioorganica Acta 2:3–13. http://www.bioorganica.org.ua/UBAdenovo/vol_9_2_ukr.htm
Stuart L (2000) Schreiber target-oriented and diversity-oriented organic synthesis in drug discovery. Science 17:1964–1969. doi:10.1126/science.287.5460.1964
Bindra JS (1973) Chapter 2 oxindole alkaloids. Alkaloids: Chem Physiol 14:83–121. doi:10.1016/S1876-0813(08)60219-5
Schun Y, Cordell GA (1985) 14\({\beta }\)-Hydroxygelsedine, a new oxindole alkaloid from Gelsemium sempervirens. J Nat Prod 48:788–791. doi:10.1021/np50041a012
Kitajima M (2007) Chemical studies on monoterpenoid indole alkaloids from medicinal plant resources Gelsemium and Ophiorrhiza. J Nat Med 61:14–23. doi:10.1007/s11418-006-0101-z
Stratmann K, Moore RE, Bonjouklian R, Deeter JB, Patterson GML, Shaffer S, Smith CD, Smitka TA (1994) Welwitindolinones, unusual alkaloids from the blue-green algae Hapalosiphon welwitschii and Westiella intricata. Relationship to fischerindoles and hapalinodoles. J Am Chem Soc 116:9935–9942. doi:10.1021/ja00101a015
James MNG, Williams GJB (1972) The molecular and crystal structure of an oxindole alkaloid (6-Hydroxy-2’-(2-methylpropyl)-3,3’ spirotetrahydropyrrolidino-oxindole). Can J Chem 50:2407–2412. doi:10.1139/v72-386
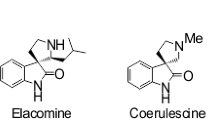
Pellegrini C, Weher M, Borschberg H-J (1996) Total synthesis of (+)-elacomine and (-)-isoelacomine, two hitherto unnamed oxindole alkaloids from Elueagnus cornrnutata. Helv Chim Acta 79:151–168. doi:10.1002/hlca.19960790116
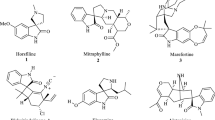
Jossang A, Jossang P, Hadi HA, Sévenet T, Bodo B (1991) Horsfiline, an oxindole alkaloid from Horsfieldia superba. J Org Chem 56:6527–6530. doi:10.1021/jo00023a016
Kornet MJ, Thio AP (1976) Oxindole-3-spiropyrrolidines and -piperidines. Synthesis and local anesthetic activity. J Med Chem 19:892–898. doi:10.1021/jm00229a007
Anderton N, Cockrum PA, Colegate SM, Edgar JA, Flower K, Vit I, Willing RI (1998) Oxindoles from Phalaris coerulescens. Phytochem 48:437–439. doi:10.1016/S0031-9422(97)00946-1
Kosuge T, Tsuj K, Hirai K, Yamaguchi K, Okamoto T, Iitaka Y (1981) Isolation and structure determination of a new marine toxin, neosurugatoxin, from the Japanese Ivory Shell. Tetrahedron Lett 2:3417–3420. doi:10.1016/S0040-4039(01)81920-1
Lerchner A, Carreira EM (2006) Synthesis of \((\pm )\)-strychnofoline via a highly convergent selective annulation reaction. Chem Eur J 12:8208–8219. doi:10.1002/chem.200600957
Beecram AF, Hart K, John SR (1968) Lambert the stereochemistry of oxindole alkaloids: uncarines A, B (formosanine), C (pteropodine), D (speciophylline), E (isopteropodine), and F. Aust J Chem 21:491–504. doi:10.1071/CH9680491
Pandey R, Singh SC, Gupta MM (2006) Heteroyohimbinoid type oxindole alkaloids from Mitragyna parvifolia. Phytochem 67:2164–2169. doi:10.1016/j.phytochem.2006.06.017
Heitzman ME, Neto CC, Winiarz E, Vaisberg AJ, Hammond GB (2005) Ethnobotany, phytochemistry and pharmacology of Uncaria (Rubiaceae). Phytochem 66:5–29. doi:10.1016/j.phytochem.2004.10.022
Kang TH, Matsumoto K, Tohda M, Murakami Y, Takayama H, Kitajima M, Aimi N, Watanabe H (2002) Pteropodine and isopteropodine positively modulate the function of rat muscarinic M\(_{1}\) and 5-HT\(_{2}\) receptors expressed in Xenopus oocyte. Eur J Pharmacol 444:39–45. doi:10.1016/S0014-2999(02)01608-4
Cui C-B, Kakeya H, Osada H (1996) Novel mammalian cell cycle lnhibitors, spirotryprostatins a and b, produced by Aspergillusfumigatus, which inhibit mammalian cell cycle at G2/M phase. Tetrahedron 52:12651–12666. doi:10.1016/0040-4020(96)00737-5
Cui C-B, Kakeya H, Osada H (1996) Spirotryprostatin B, a novel mammalian cell cycle inhibitor produced by Aspergillus fumigatus. J Antibiot (Tokyo) 49:832–835. doi:10.7164/antibiotics.49.832
Ket D, Lu Y, Nikolovska-Coleska Z, Wang G, Qiu S, Shangary S, Gao W, Qin D, Stuckey J, Krajewski K, Roller PP, Wang S (2006) Structure-based design of spiro-oxindoles as potent, specific small-molecule inhibitors of the MDM2-p53 interaction. J Med Chem 49:3432–3435. doi:10.1021/jm051122a
Yu S, Qin D, Shangary S, Chen J, Wang G, Ding K, McEachern D, Qiu S, Nikolovska-Coleska Z, Miller R, Kang S, Yang D, Wang S (2009) Potent and orally active small-molecule inhibitors of the MDM2-p53 interaction. J Med Chem 52:7970–7973. doi:10.1021/jm901400z
Williams RM, Cox RJ (2003) Paraherquamides, brevianamides, and asperparalines: laboratory synthesis and biosynthesis. An Interim Rep. Acc Chem Res 36:127–139. doi:10.1021/ar020229e
Sunderhaus JD, Sherman DH, Williams RM (2011) Studies on the biosynthesis of the stephacidin and notoamide natural products: a stereochemical and genetic conundrum. Israel J Chem 51:442–452. doi:10.1002/ijch.201100016
Birch AJ, Wright JJ (1969) The brevianamides: a new class of fungal alkaloid. J Chem Soc Chem Commun 12:644–645. doi:10.1039/C2969000644B
Paterson RRM, Simmonds MJS, Kemmelmeier C, Blaney WM (1990) Effects of brevianamide A, its photolysis product brevianamide D, and ochratoxin A from two Penicillium strains on the insect pests Spodoptera frugiperda and Heliothis virescens. Mycol Res 94:538–542. doi:10.1016/S0953-7562(10)80017-6
Auclair K, Sutherland A, Kennedy J, Witter DJ, Van den Heever JP, Hutchinson CR, Vederas JC (2000) Lovastatin nonaketide synthase catalyzes an intramolecular diels-alder reaction of a substrate analogue. J Am Chem Soc 122:11519–11520. doi:10.1021/ja003216+
Greshock TJ, Grubbs AW, Jiao P, Wicklow DT, Gloer JB, Williams RM (2008) Isolation, structure elucidation, and biomimetic total synthesis of versicolamide B, and the isolation of antipodal (-)-stephacidin A and (+)-notoamide B from aspergillus versicolor NRRL 35600. Angew Chem Int Ed 47:3573–3577. doi:10.1002/anie.200800106
Takasugi M, Monde K, Katsui N, Shirata A (1987) Spirobrassinin, a novel sulfur-contaning phytoalexin from the daikon Raphanus sati6us L. var. hortensis (Cruciferae). Chem Lett 16:1631–1632. doi:10.1246/cl.1987.1631
Suchý M, Kutschy P, Monde K, Goto H, Harada N, Takasugi M, Dzurilla M, Balentová E (2001) Synthesis, absolute configuration, and enantiomeric enrichment of a cruciferous oxindole phytoalexin, (s)-(-)-spirobrassinin, and its oxazoline analog. J Org Chem 66:3940–3947. doi:10.1021/jo0155052
Monde K, Taniguchi T, Miura N, Kutschy P, Curillová Z, Pilátová M, Mojzis J (2005) Chiral cruciferous phytoalexins: preparation, absolute configuration, and biological activity. Bioorg Med Chem 13:5206–5212. doi:10.1016/j.bmc.2005.06.001
Monde K, Takasugi M, Shirata A (1995) Three sulphur-containing stress metabolites from Japanese radish. Phytochem 39:581–586. doi:10.1016/0031-9422(95)00011-U
Marti C, Erick M, Carreira EM (2003) Construction of spiro[pyrrolidine-3,3’-oxindoles] recent applications to the synthesis of oxindole alkaloids. Eur J Org Chem 2003:2209–2219. doi:10.1002/ejoc.200300050
Li Sh-M (2010) Prenylated indole derivatives from fungi: structure diversity, biological activities, biosynthesis and chemoenzymatic synthesis. Nat Prod Rep 27:57–78. doi:10.1039/b909987p
Hart DJ (2010) The spiroquinazoline family of alkaloids: a review. ARKIVOC IV: 32-65.doi:10.3998/ark.5550190.0011.405
Singh GS, Desta ZY (2012) Isatins as privileged molecules in design and synthesis of spiro-fused cyclic frameworks. Chem Rev 112:6104–6155. doi:10.1021/cr300135y
Cheng D, Ishihara Y, Tan B, Barbas CF III (2014) Organocatalytic asymmetric assembly reactions: synthesis of spirooxindoles via organocascade strategies. ACS Catal 4:743–762. doi:10.1021/cs401172r
Zhen Yajun, Colin MT, Singh Suresh B (2014) The use of spirocyclic scaffolds in drug discovery. Bioorg Med Chem Lett 24:3673–3682. doi:10.1016/j.bmcl.2014.06.081
Wenkert E, Delhofen JHU, Bhaitacharyya NK (1959) 3-Hydroxymethyleneoxindole and its derivatives. J Am Chem Soc 81:3763–3768. doi:10.1021/ja01523a068
Laus G (1998) Kinetics of isomerization of tetracyclic spiro oxindole alkaloids. J Chem Soc, Perkin Trans 2:315–317. doi:10.1039/A705871C
Miyake FY, Yakushijin K, Horne DA (2004) Preparation and synthetic applications of 2-halotryptamines: synthesis of elacomine and isoelacomine. Org Lett 6:711–713. doi:10.1021/ol030138x
Nussbaum F, Danishefsky SJ (2000) A rapid total synthesis of spirotryprostatin B: proof of its relative and absolute stereochemistry. Angew Chem Int Ed 29:2175–2178. doi:10.1002/15213773(20000616)39:12<2175::AID-ANIE2175>3.0.CO;2-J
Finch N, Taylor WI (1962) Oxidative transformations of indole alkaloids. I. The preparation of oxindoles from yohimbine; the structures and partial syntheses of mitraphylline, rhyncophylline and corynoxeine. J Am Chem Soc 84:1318–1320. doi:10.1021/ja00866a062
White JD, Li Y, Ihle DC (2010) Tandem intramolecular photocycloaddition-retro-mannich fragmentation as a route to spiro[pyrrolidine-3,3’-oxindoles]. total synthesis of \((\pm )\)-coerulescine, \((\pm )\)-horsfiline, \((\pm )\)-elacomine, and \((\pm )\)-6-deoxyelacomine. J Org Chem 75:3569–3577. doi:10.1021/jo1002714
Wang H, Ganesan A (2000) A biomimetic total synthesis of (-)-spirotryprostatin B and related studies. J Org Chem 65:4685–4693. doi:10.1021/jo000306o
Peterson AC, Cook JM (1995) Studies directed toward the enantiospecific synthesis of Gardneria, Voacanga, and Alstonia oxindole alkaloids. J Org Chem 60:120–129. doi:10.1021/jo00106a024
Yu P, Cook JM (1997) Diastereospecific synthesis of ketooxindoles. Potential intermediates for the synthesis of alstonisine as well as for Voachalotine related oxindole alkaloids. Tetrahedron Lett 38:8799–8802. doi:10.1016/S0040-4039(97)10420-8
Somei M, Noguchi K, Yamagami R, Kawada Y, Yamada K, Yamada F (2000) Preparation and a novel rearrangement reaction of 1,2,3,4-tetrahydro-9-hydroxy-\({\beta }\)-carboline, and their applications for the total synthesis of \((\pm )\)-coerulescine. Heterocycl 53:7–10. doi:10.3987/COM-99-8743
Dounay AB, Overman LE (2003) The asymmetric intramolecular heck reaction in natural product total synthesis. Chem Rev 103:2945–2963. doi:10.1021/cr020039h
Kamisaki H, Nanjo T, Tsukano C, Takemoto Y (2011) Domino Pd-catalyzed heck cyclization and bismuth-catalyzed hydroamination: formal synthesis of elacomine and isoelacomine. Chem Eur J 17:626–633. doi:10.1002/chem.201002287
Kamisaki H, Yasui Y, Takemoto Y (2009) Pd-catalyzed intramolecular amidation of 2-(buta-1,3-dienyl)phenylcarbamoyl chloride: a concise synthesis of spiro[indoline-3,3\(^\prime \)-pyrrolidine]. Tetrahedron Lett 50:2589–2592. doi:10.1016/j.tetlet.2009.03.100
Overman LE, Rosen MD (2000) Total synthesis of (-)-spirotryprostatin B and three stereoisomers. Angew Chem Int Ed 39:4596–4599. doi:10.1002/15213773(20001215)39:24<4596::AID-ANIE4596>3.0.CO;2-F
Jaegli S, Dufou J, Wei H, Piou T, Duan X, Vors J, Neuville L, Zhu J (2010) Palladium-catalyzed carbo-heterofunctionalization of alkenes for the synthesis of oxindoles and spirooxindoles. Org Lett 12:4498–4501. doi:10.1021/ol101778c
Jaegli S, Erb W, Retailleau P, Vors J-P, Neuville L, Zhu J (2010) Palladium-catalyzed domino process to spirooxindoles: ligand effect on aminopalladation versus carbopalladation. Chem Eur J 16:5863–5867. doi:10.1002/chem.201000312
Jaegli S, Vors JP, Neuville L, Zhu J (2010) Palladium-catalyzed domino Heck/cyanation: synthesis of 3-cyanomethyloxindoles and their conversion to spirooxoindoles. Tetrahedron 66:8911–8921. doi:10.1016/j.tet.2010.09.056
Deppermann N, Thomanek H, Prenzel A, Maison W (2010) Pd-catalyzed assembly of spirooxindole natural products: a short synthesis of horsfiline. J Org Chem 75:5994–6000. doi:10.1021/jo101401z
Cravotto G, Giovenzana GB, Pilati T, Sisti M, Palmisano G (2001) Azomethine ylide cycloaddition/reductive heterocyclization approach to oxindole alkaloids: asymmetric synthesis of (-)-horsfiline. J Org Chem 66:8447–8453. doi:10.1021/jo015854w
Grigg R, Basanagoudar LD, Kennedy DA, Malone JF, Thianpatanagul S (1982) X=Y-ZH systems as potential 1,3-dipoles. Cycloadditions of thiominoethers and thioiminocarbonates. Tetrahedron Lett 23:2803–2806. doi:10.1016/S0040-4039(00)87463-8
Fejes I, Nyerges M, Nyerges M, Szöllõsy Á, Blaskó G, Tõke L (2001) 2-Oxoindolin-3-ylidene derivatives as 2-\(\pi \) components in 1,3-dipolar cycloadditions of azomethine ylides. Tetrahedron 57:1129–1137. doi:10.1016/S0040-4020(00)01085-1
Sebahar PR, Osada H, Usuib T, Williams RM (2002) Asymmetric, stereocontrolled total synthesis of (+) and (-)-spirotryprostatin B via a diastereoselective azomethine ylide [1,3]-dipolar cycloaddition reaction. Tetrahedron 58:6311–6322. doi:10.1016/S0040-4020(02)00630-0
Bell SEV, Brown RFC, FrW Eastwood, Horvath JM (2000) An approach to some spiro oxindole alkaloids through cycloaddition reactions of 3-methylideneindolin-2-one. Aust J Chem 53:183–190. doi:10.1002/chin.200043209
Onishi T, Sebahar PR, Williams RM (2004) Concise, asymmetric total synthesis of spirotryprostatin A. Tetrahedron 60:9503–9515. doi:10.1016/j.tet.2004.07.047
Fejes I, To’ke L, Nyerges M, Pak ChS (2000) Tandem in situ generation of azomethine ylides and base sensitive nitroethylene dipolarophiles. Tetrahedron 56:639–644. doi:10.1016/S0040-4020(99)01028-5
Serov AB, Kartsev VG, Aleksandrov YuA, Dolgushin FM (2005) 1,3-Dipolar cycloaddition reaction of heteroaromatic N-ylides with 3-[(E)-2-aryl(hetaryl)-2-oxoethylidene]indolin-2-ones. Russ Chem Bull 54:2432–2436. doi:10.1007/s11172-006-0133-2
Lo M, Neumann CS, Nagayama S, Perlstein EO, Schreiber SL (2004) A library of spirooxindoles based on a stereoselective three-component coupling reaction. J Am Chem Soc 126:16077–16086. doi:10.1021/ja045089d
Ding K, Lu Y, Nikolovska-Coleska Z, Wang G, Qiu S, Shangary S, Gao W, Qin D, Stuckey J, Krajewski K, Roller PP, Wang S (2006) Structure-based design of spiro-oxindoles as potent, specific small- molecule inhibitors of the MDM2-p53 interaction. J Med Chem 49:3432–3435. doi:10.1021/jm051122a
Ding K, Lu Y, Nikolovska-Coleska Z, Qiu S, Ding Y, Gao W, Stuckey J, Krajewski K, Roller P, Tomita Y, Parrish D, Deschamps J,Wang S (2005) Structure-based design of potent non-peptide MDM2 inhibitors. J Am Chem Soc 127:10130–10131. doi:10.1021/ja051147z
Ding K, Wang G, Deschamps JR, Parrish DA, Wang Sh (2005) Synthesis of spirooxindoles via asymmetric 1,3-dipolar cycloaddition. Tetrahedron Lett 46:5949–5951. doi:10.1016/j.tetlet.2005.06.114
Chen Xiao-Hua, Wei Qiang, Luo Shi-Wei, Xiao Han, Gong Liu-Zhu (2009) Organocatalytic synthesis of spiro[pyrrolidin-3,3\(^{\prime }\)-oxindoles] with high enantiopurity and structural diversity. J Am Chem Soc 131:13819–13825. doi:10.1021/ja905302f
Lashgari N, Ziarani GM (2012) Synthesis of heterocyclic compounds based on isatin through 1,3-dipolar cycloaddition reactions. ARKIVOC I :277–320. doi:10.3998/ark.5550190.0013.108
Rizzi GP (1970) Evidence for an azomethine ylide intermediate in the carbonyl-assisted decarboxylation dl-phenylephrine hydrochloride of sarcosine. A novel synthesis of dl-phenylephrine hydrochloride. J Org Chem 35:2069–2072. doi:10.1021/jo00831a098
Da Silva JFM, Garden SJ, Pinto AC (2001) The chemistry of isatins: a review from 1975 to 1999. J Braz Chem Soc 12:273–324. doi:10.1590/S0103-50532001000300002
Coulter T, Grigg R, Maloneb JF, Sridharan V (1991) Chiral induction in cycloaddition reactions of azomethine ylides derived from secondary \({\alpha }\)-amino acids by the decarboxylative route. Tetrahedron Lett 32:5417–5420. doi:10.1016/S0040-4039(00)92401-8
Grigg R (1987) Prototropic routes to 1,3- and 1,5-dipoles, and 1,2-ylides: applications to the synthesis of heterocyclic compounds. Chem Soc Rev 16:89–121. doi:10.1039/CS9871600089
Fokas D, Ryan WJ, Casebier DS, Coffen DL (1998) Solution phase synthesis of a spiro[pyrrolidine-2,3\(^{\prime }\)-oxindole] library via a three component 1,3-dipolar cycloaddition reaction. Tetrahedron Lett 39:2235–2238. doi:10.1016/S0040-4039(98)00234-2
Powers DG, Casebier DS, Fokas D, Ryan WJ, Troth JR, Coffen DL (1998) Automated parallel synthesis of chalcone-based screening libraries. Tetrahedron 54:4085–4096. doi:10.1016/S0040-4020(98)00137-9
Pardasani RT, Pardasani P, Chaturvedi V, Yadav SK, Saxena A, Sharma I (2003) Theoretical and synthetic approach to novel spiroheterocycles derived from isatin derivatives and L-proline via 1,3-dipolar cycloaddition. Heteroatom Chem 14:36–41. doi:10.1002/hc.10063
Sarrafi Y, Hamzehloueian M, Alimohammadi K, Yeganegi S (2011) An experimental and theoretical investigation of the regio- and stereoselectivity of the polar [3+2] cycloaddition of azomethine ylides to nitrostyrene. Tetrahedron 67:1589–1597. doi:10.1016/j.tet.2010.12.034
Chen G, Miao Y, Zhou R, Zhang L, Zhang Y, Hao X (2013) Investigation of regioselectivity in the synthesis of spiro[pyrrolidine-2,30-oxindoles] by use of the Huisgen reaction. Res Chem Intermed 39:2445–2450. doi:10.1007/s11164-012-0770-z
Sarrafi Y, Hamzehloueian M, Alimohammadi K, Yeganegi S (2012) Experimental and theoretical approaches to [1,5]-prototropic generation of an azomethine ylide and a 1,3-dipolar cycloaddition for novel spiropyrrolidine oxindoles synthesis. J Mol Struct 1030:168–176. doi:10.1016/j.molstruc.2012.04.013
Rehn S, Bergman J, Stainsland B (2004) The three-component reaction between isatin, \({\alpha }\)-amino acids, and dipolarophiles. Eur J Org Chem 2:413–418. doi:10.1002/ejoc.200300621
Chen G, He H, Ding J, Hao X (2009) Synthesis and antitumor activity evaluation of regioselective spiro[pyrrolidine-2,3’-oxindole] compounds. Heterocycl Commun 15:355–360. doi:10.1515/HC.2009.15.5.355
Hemamalini A, Nagarajan S, Ravinder P, Subramanian V, Thangamuthu B, Das M (2011) An easy access to novel sugar-based spirooxindole-pyrrolidines or -pyrrolizidines through [3+2] cycloaddition of azomethine ylides. Synth 15:2495–2504. doi:10.1055/s-0030-1260111
Hemamalini A, Nagarajan S, Das ThM (2012) A novel class of sugar-based ether-linked-dispirooxindolo-pyrrolidines/pyrrolizidines through [3+2]-cycloaddition of azomethine ylides. Carbohydr Res 352:12–17. doi:10.1016/j.carres.2012.01.023
Wu G, Ouyang L, Liu J, Zeng S, Huang W, Han B, Wu F, He G, Xiang M (2013) Synthesis of novel spirooxindolo-pyrrolidines, pyrrolizidines, and pyrrolothiazoles via a regioselective three-component [3+2] cycloaddition and their preliminary antimicrobial evaluation. Mol Divers 17:271–283. doi:10.1007/s11030-013-9432-3
Kanagaraju G, Thangamani A (2014) Design and synthesis of spiro derivatives containing a thiophene ring and evaluation of their anti-microbial activity. Orient J Chem 30:1619–1630. doi:10.13005/ojc/300421
Azizian J, Asadi A, Jadidi Kh (2001) One-pot highly diastereo-selective synthesis of new 2-substituted 8-(sprio-3\(^\prime \)-indolino-2\(^\prime \)-one)-pyrrolo[3,4-a]-pyrrolizine-1,3-diones mediated by azomethine ylide induced by microwave irradiation. Synth Commun 31:2727–2733. doi:10.1081/SCC-100105318
Girgis AS, Stawinski J, Ismail NSM, Fara H (2012) Synthesis and QSAR study of novel cytotoxic spiro[3H-indole-3,2’(1\(^{\prime }\)H)-pyrrolo[3,4-c]pyrrole]-2,3\(^{\prime }\),5\(^{\prime }\)(1H,2\(^{\prime }\)H,4\(^{\prime }\)H)-triones. Eur J Med Chem 47:312–322. doi:10.1016/j.ejmech.2011.10.058
Pavlovskaya TL, Red’kin RG, Yaremenko FG, Shishkina SV, Shishkin OV, Musatov VI, Lipson VV (2013) Synthesis and chemical properties of new derivatives of 3a\(^\prime \),6a\(^\prime \)-dihydro-2\(^\prime \)H-spiro- [indole-3,1\(^\prime \)-pyrrolo[3,4-c]pyrrole]- 2,4\(^\prime \),6\(^\prime \)(1H,3\(^\prime \)H,5\(^\prime \)H)-trione. Chem Heterocycl Comp 49: 882–896 (Russian Original 49: 945–960). doi:10.1007/s10593-013-1322-1
Karthikeyan K, Sivakumar PM, Doble M, Perumal PT (2010) Synthesis, antibacterial activity evaluation and QSAR studies of novel dispiropyrrolidines. Eur J Med Chem 45:3446–3452. doi:10.1016/j.ejmech.2010.04.035
Faraji L, Arvinnezhad H, Alikami N, Jadidi K (2010) Synthesis of pyrrolizidine derivatives in ionic liquid [bmim]Br. Lett Org Chem 7:472–415. doi:10.2174/157017810791824946
Pavlovskaya TL, Lipson VV, Yaremenko FG, Musatov VI (2013) Acryl- and methacrylamides. New dipolarophiles in reactions of [2+3]-dipolar cycloaddition to 2-oxindolazomethine ylides. Russ J Org Chem 49(11):1712–1714 (Russian Original 49:1728–1730). doi:10.1134/S1070428013110274
Pavlovskaya TL, Yaremenko FG, Lipson VV, Shishkina SV, Shishkin OV, Musatov VI, Karpenko AS (2014) The regioselective synthesis of spirooxindolo pyrrolidines and pyrrolizidines via three-component reactions of acrylamides and aroylacrylic acids with isatins and \({\alpha }\)-amino acids. Beilstein J Org Chem 10:117–126. doi:10.3762/bjoc.10.8
Xie Yong-Mei, Yao Yu-Qin, Sun Hong-Bao, Yan Ting-Ting, Liu Jie, Kang Tai-Ran (2011) Facile Synthesis of Functionalized Spiropyrrolizidine Oxindoles via a Three-Component Tandem Cycloaddition Reaction. Molecules 16:8745–8757. doi:10.3390/molecules16108745
Murugan R, Raghunathan R, Narayanan SS (2010) Synthesis of novel spiroheterocycles through 1,3-dipolar cycloaddition of azomethine ylides with triarylideneacetylacetone through decarboxylation. Synth Commun 40:3135–3151. doi:10.1080/00397910903341189
Ghandi M, Taheri A, Abbasi A (2010) A facile synthesis of chromeno[3,4-c]spiropyrrolidine-oxindoles via 1,3-dipolar cycloadditions. Tetrahedron 66:6744–6748. doi:10.1016/j.tet.2010.06.078
Liu H, Dou G, Shi D (2010) Regioselective synthesis of novel spiropyrrolidines and spirothiapyrrolizidines through multicomponent 1,3-dipolar cycloaddition reaction of azomethine ylides. J Comb Chem 12:633–637. doi:10.1021/cc100035q
Rao JNS, Raghunathan R (2012) An expedient diastereoselective synthesis of pyrrolidinyl spirooxindoles fused to sugar lactone via [3+2] cycloaddition of azomethine ylides. Tetrahedron Lett 53:854–858. doi:10.1016/j.tetlet.2011.12.025
Shanmugam P, Viswambharan B, Madhavan S (2007) Synthesis of novel functionalized 3-spiropyrrolizidine and 3-spiropyrrolidine oxindoles from baylis-hillman adducts of isatin and heteroaldehydes with azomethine ylides via [3+2]-cycloaddition. Org Lett 9:4095–4098. doi:10.1021/ol701533d
Chen H, Wang S, Xu X, Ji SJ (2011) Facile three-component synthesis of spirooxindolepyrrololine ring systems via 1,3-dipolar cycloaddition with 1,4-naphthoquinone. Synth Commun 41:3280–3288. doi:10.1080/00397911.2010.517413
Bhaskar G, Arun Y, Balachandran C, Paramasivan CS, Perumal T (2012) Synthesis of novel spirooxindole derivatives by one pot multicomponent reaction and their antimicrobial activity. Eur J Med Chem 51:79–91. doi:10.1016/j.ejmech.2012.02.024
Taghizadeh MJ, Arvinnezhad H, Samadi S, Jadidi K, Javidan A, Notash B (2012) Synthesis of new enantiomerically pure spirooxindolopyrrolizidines via a three-component asymmetric 1,3-dipolar cycloaddition reaction of azomethine ylides derived from isatin. Tetrahedron Lett 53:5148–5150. doi:10.1016/j.tetlet.2012.07.066
Lakshmi NV, Thirumurugan P, Jayakumar C, Paramasivan T (2010) An easy access to novel spiro-fused pyrrolo benzo[b]thiophene 1,1-dioxide derivatives via 1,3-dipolar cycloaddition using benzo[b]thiophene 1,1-dioxide. Synlett 6:955–961. doi:10.1055/s-0029-1219550
Lakshmi NV, Thirumurugan P, Perumal PT (2010) An expedient approach for the synthesis of dispiropyrrolidine bisoxindoles, spiropyrrolidine oxindoles and spiroindane-1,3-diones through 1,3-dipolar cycloaddition reactions. Tetrahedron Lett 51:1064–1068. doi:10.1016/j.tetlet.2009.12.079
Jain AK, Bhati DS (2011) Direct construction of novel dispiro heterocycles through 1,3-dipolar cycloaddition of azomethine ylides. Tetrahedron Lett 52:5333–5337. doi:10.1016/j.tetlet.2011.08.014
Jayashankaran J, Manian DRS, Raghunathan R (2004) A facile synthesis of novel dispiroheterocycles through solvent-free microwave-assisted [3+2] cycloaddition of azomethine ylides. Tetrahedron Lett 45:7303–7305. doi:10.1016/j.tetlet.2004.08.015
Babu S, Raghunathan R (2007) Ultrasonic assisted-silica mediated [3+2] cycloaddition of azomethine ylides - a facile multicomponent one-pot synthesis of novel dispiroheterocycles. Tetrahedron Lett 48:6809–6813. doi:10.1016/j.tetlet.2007.07.085
Jain R, Sharma K, Kumar D (2012) Ionic liquid mediated 1,3-dipolar cycloaddition of azomethine ylides: a facile and green synthesis of novel dispiro heterocycles. Tetrahedron Lett 53:1993–1997. doi:10.1016/j.tetlet.2012.02.029
Maheswari SU, Balamurugan K, Perumal S, Yogeeswari P, Sriram D (2010) A facile 1,3-dipolar cycloaddition of azomethine ylides to 2-arylidene-1,3-indanediones: synthesis of dispiro-oxindolylpyrrolothiazoles and their antimycobacterial evaluation. Bioorg Med Chem Lett 20:7278–7282. doi:10.1016/j.bmcl.2010.10.080
El-Ahl Abdel-Aziz S (2002) Three-component 1,3-dipolar cycloaddition reactions in synthesis of spiro[pyrrolidine-2,30-oxindoline] derivatives. Heteroat Chem 13:324–329. doi:10.1002/hc.10038
Raj AA, Raghunathan R (2001) A novel entry into a new class of spiroheterocyclic framework: regioselective synthesis of dispiro[oxindole-cyclohexanone]pyrrolidines and dispiro[oxindole-hexahydroindazole]pyrrolidines. Tetrahedron 57:10293–10298. doi:10.1016/S0040-4020(01)01042-0
Girgis AS (2009) Regioselective synthesis and stereochemical structure of anti-tumor active dispiro[3H-indole-3,2\(^{\prime }\)-pyrrolidine-3\(^{\prime },3^{\prime \prime }\)-piperidine]-2(1H), 4\(^{\prime \prime }\)-diones. Eur J Med Chem 44:1257–1264. doi:10.1016/j.ejmech.2008.09.007
Hazra A, Paira P, Sahu KB, Naskar S, Saha P, Paira R, Mondal S, Maity A, Luger P, Weber M, Mondal NB, Banerjee S (2010) Chemistry of andrographolide: formation of novel di-spiropyrrolidino and di-spiropyrrolizidino-oxindole adducts via one-pot three-component [3+2] azomethine ylide cycloaddition. Tetrahedron Lett 51:1585–1588. doi:10.1016/j.tetlet.2010.01.052
Babu SR, Raghunathan R (2008) An easy access to novel steroidal dispiropyrrolidines through 1,3-dipolar cycloaddition of azomethine ylides. Tetrahedron Lett 49:4618–4620. doi:10.1016/j.tetlet.2008.05.089
Bharitkar YP, Kanhar S, Suneel N, Mondal SK, Hazra A, Mondal NB (2015) Chemistry of withaferin-A: chemo, regio, and stereoselective synthesis of novel spiro-pyrrolizidino-oxindole adducts of withaferin-A via one-pot three-component [3+2] azomethine ylide cycloaddition and their cytotoxicity evaluation. Mol Divers 19:251–261. doi:10.1007/s11030-015-9574-6
Poornachandran M, Raghunathan R (2006) Synthesis of dispirooxindolecycloalka[d]pyrimidino[2,3-b]-thiazole pyrrolidine/thiapyrrolizidine ring systems. Tetrahedron 62:11274–11281. doi:10.1016/j.tet.2006.09.008
Murugan R, Anbazhagan S, Narayanan SS (2009) Synthesis and in vivo antidiabetic activity of novel dispiropyrrolidines through [3+2] cycloaddition reactions with thiazolidinedione and rhodanine derivatives. Eur J Med Chem 44:3272–3279. doi:10.1016/j.ejmech.2009.03.035
Liu H, Zou Y, Hu Y, Shi DQ (2011) An efficient one-pot synthesis of dispiropyrrolidine derivatives through 1,3-dipolar cycloaddition reactions under ultrasound irradiation. J Heterocycl Chem 48:877–881. doi:10.1002/jhet.654
Hu Y, Zou Y, Wu H, Shi D (2012) A facile and efficient ultrasound-assisted synthesis of novel dispiroheterocycles through 1,3-dipolar cycloaddition reactions. Ultrason Sonochem 19:264–269. doi:10.1016/j.ultsonch.2011.07.006
Mamari KA, Ennajih H, Zouihri H, Bouhfid R, Ng SW, Essassi EM (2012) Synthesis of novel dispiro-oxindoles via 1,3-dipolar cycloaddition reactions of azomethine ylides. Tetrahedron Lett 53:2328–2331. doi:10.1016/j.tetlet.2012.02.097
Dalpozzo R, Bartoli G, Bencivenni G (2012) Recent advances in organocatalytic methods for the synthesis of disubstituted 2- and 3-indolinones. Chem Soc Rev 41:7247–7290. doi:10.1039/C2CS35100E
Tan B, Zeng X, Leong WWY, Shi Barbas III CF, Zhong G (2012) Core structure-based design of organocatalytic [3+2]-cycloaddition reactions: highly efficient and stereocontrolled syntheses of 3,3\(^{\prime }\)-pyrrolidonyl spirooxindoles. Chem Eur J 18:63–67. doi:10.1002/chem.201103449
Cao Y, Jiang X, Liu L, Shen FF, Zhang F, Wang R (2011) Enantioselective michael/cyclization reaction sequence: scaffold-inspired synthesis of spirooxindoles with multiple stereocenters. Angew Chem 123:9290–9293. doi:10.1002/ange.201104216
Hande SM, Nakajima M, Kamisaki H, Tsukano C, Takemoto Y (2011) Flexible strategy for syntheses of spirooxindoles using palladium- catalyzed carbosilylation and sakurai-type cyclization. Org Lett 13:1828–1831. doi:10.1021/ol2003447
Alcaide B, Almendros P, Rodriguez-Acebes R (2006) Efficient entry to diversely functionalized spirocyclic oxindoles from isatins through carbonyl-addition/cyclization reaction sequences. J Org Chem 71:2346–2351. doi:10.1021/jo0525027
Du D, Hu Z, Jin J, Lu Y, Tang W, Wang B, Lu T (2012) N-Heterocyclic carbene-catalyzed three-component domino reaction of alkynyl aldehydes with oxindoles. Org Lett 14:1274–1277. doi:10.1021/ol300148f
Castaldi MP, Troast DM, Porco JA (2009) Stereoselective synthesis of spirocyclic oxindoles via Prins cyclizations. J Org Lett 11:3362–3365. doi:10.1021/ol901201k
Zhang Y, Panek JS (2009) Stereocontrolled Synthesis of spirooxindoles through lewis acid-promoted [5+2]-annulation of chiral silyl alcohols. Org Lett 11:3366–3369. doi:10.1021/ol901202t
Wang J, Crane EA, Scheidt KA (2011) Highly stereoselective Brønsted acid catalyzed synthesis of spirooxindole pyrans. Org Lett 13:3086–3089. doi:10.1021/ol200987c
Zh L, Shi M (2012) Nitrogen- and phosphorus-containing Lewis base catalyzed [4+2] and [3+2] annulation reactions of isatins with but-3-yn-2-one. Eur J Org Chem 3:581–586. doi:10.1002/ejoc.201101338
Zhu SL, Ji SJ, Zhang Y (2007) A simple and clean procedure for three-component synthesis of spirooxindoles in aqueous medium. Tetrahedron 63:9365–9372. doi:10.1016/j.tet.2007.06.113
Chen WB, Wu ZJ, Pei QL, Cun LF, Zhang XM, Yuan WC (2010) Highly enantioselective construction of spiro[4h-pyran-3,3’-oxindoles] through a domino Knoevenagel/Michael/cyclization sequence catalyzed by cupreine. Org Lett 12:3132–3135. doi:10.1021/ol1009224
Shishkina SV, Shishkin OV, Redkin RG, Shemchuk LA, Chernykh VP (2007) Crystal structure of 3,4-spiro-[(5-acetyl-2-amino-3-carbethoxy-6-methyl-4H-pyrano)-1H,3H-indol-2-on]. Acta Cryst 63:3193–3196. doi:10.1107/S1600536807027547
Shemchuk LA, Chernykh VP, Redkin RG (2008) Synthesis of fused 2\(^{\prime }\)-amino-3\(^{\prime }\)-R-spiro-[indole-3,4\(^{\prime }\)-pyran]-2(1H)-ones. Russ J Org Chem 44:1789–1791. doi:10.1134/S1070428008120117
Litvinov YM, Mortikov VY, Shestopalov AM (2008) Versatile three-component procedure for combinatorial synthesis of 2-aminospiro[(3\(^{\prime }\)h)-indol-3\(^{\prime }\),4-(4h)-pyrans]. J Comb Chem 10:741–745. doi:10.1021/cc800093q
Redkin RG, Shemchuk LA, Chernykh VP, Shishkin OV, Shishkina SV (2007) Synthesis and molecular structure of spirocyclic 2-oxindole derivatives of 2-amino-4H-pyran condensed with the pyrazolic nucleus. Tetrahedron 63:11444. doi:10.1016/j.tet.2007.08.050
Heravi MH, Zakeri M, Moharami A (2012) Versatile three-component procedure for combinatorial synthesis of spiro-oxindoles with fused chromenes catalysed by L-proline. J Chem Sci 124:865–869. doi:10.1007/s12039-012-0284-7
Wang L-M, Jiao N, Qiu J, Yu J-J, Liu J-Q, Guo F-L, Liu Y (2010) Sodium stearate-catalyzed multicomponent reactions for efficient synthesis of spirooxindoles in aqueous micellar media. Tetrahedron 66:339–343. doi:10.1016/j.tet.2009.10.091
Rad-Moghadam K, Youseftabar-Miri L (2011) Ambient synthesis of spiro[4H-pyran-oxindole] derivatives under [BMIm]BF\(_{4}\) catalysis. Tetrahedron 67:5693–5699. doi:10.1016/j.tet.2011.05.077
Mobinikhaledi A, Foroughifar N, Fard MAB (2011) Simple and efficient method for three-component synthesis of spirooxindoles in aqueous and solvent-free media. Synth Commun: Int J Rap Commun Synth Org Chem 41:441–450. doi:10.1080/00397911003587507
Zhao L-Q, Zhou B, Li Y-Q (2011) An efficient one-pot three-component reaction for synthesis of spirooxindole derivatives in water media under catalyst-free condition. Heteroat Chem 22:673–677. doi:10.1002/hc.20723
Elinson MN, Ilovaisky AI, Merkulova VM, Zaimovskaya TA, Nikishin GI (2012) Non-catalytic thermal multicomponent assembling of isatin, cyclic CH-acids and malononitrile: an efficient approach to spirooxindole scaffold. Mendeleev Commun 22:143–144. doi:10.1016/j.mencom.2012.05.010
Hasaninejada A, Golzar N, Beyrati M, Zare A, Doroodmand MM (2013) Silica-bonded 5-n-propyl-octahydro-pyrimido[1,2-a]azepinium chloride (SB-DBU)Cl as a highly efficient, heterogeneous and recyclable silica-supported ionic liquid catalyst for the synthesis of benzo[b]pyran, bis(benzo[b]pyran) and spiro-pyran derivatives. J Mol Catal A: Chem 372:137–150. doi:10.1016/j.molcata.2013.02.022
Liu Y, Ren Z, Cao W, Chen J, Deng H, Shao M (2011) Solvent-free one-pot synthesis of spiro[indoline-3,4\(^{\prime }(1\text{ H }^{\prime })\)-pyrano[2,3-c]pyrazol]-2-one derivatives by grinding. Synth Commun 41:3620–3626. doi:10.1080/00397911.2010.519449
Zou Y, Hu Y, Liu H, Shi D (2012) Rapid and efficient ultrasound-assisted method for the combinatorial synthesis of spiro[indoline-3,4\(^{\prime }\)-pyrano[2,3-c]pyrazole] derivatives. ACS Comb Sci 14:38–43. doi:10.1021/co200128k
Ukrainets IV, Redkin RG, Sidorenko LV, Turov AV (2009) 4-Hydroxy-2-quinolones 172. Synthesis and structure of 4,3\(^\prime \)-spiro[(6-allyl-2-amino-5-oxo-5,6-dihydro-4h-pyrano-[3,2-c]quinoline-3-carbo-nitrile)-2\(^\prime \)-oxindole]. Chem Heterocycl Comp 45:1478–1484. doi:10.1007/s10593-010-0454-9
Ghahremanzadeh R, Amanpour T, Bazgir A (2009) An efficient, three-component synthesis of spiro[benzo[g]chromene-4,3\(^{\prime }\)-indoline]-3-carbonitrile and spiro[indoline-3,5\(^{\prime }\)-pyrano[2,3-d]pyrimidine]-6\(^{\prime }\)-carbonitrile derivatives. J Heterocycl Chem 46:1266–1270. doi:10.1002/jhet.240
Zhao H, Lan Y-B, Liu Z-M, Wang Y, Wang X-W, Tao J-C (2012) Enantioselective construction of spiro[2H-pyran-3,4’-indoline] by a systematic Michael/Reduction/Cyclization sequence triggered by the asymmetric conjugate addition of ketones to isatylidenemalononitriles. Eur J Org Chem 10:1935–1944. doi:10.1002/ejoc.201101810
Liang B, Kalidindi S, Porco JA, Stephenson CRJ (2010) Multicomponent reaction discovery: three-component synthesis of spirooxindoles. Org Lett 12:572–575. doi:10.1021/ol902764k
Ghahremanzadeh R, Fereshtehnejad F, Yasaei Z, Amanpour T, Bazgir A (2010) One-pot and three-component synthesis of spiro[chromeno[2,3-d]pyrimidine-5,30-indoline]-diones and spiro[chromeno[2,3-c]pyrazole-4,30-indoline]-diones. J Heterocycl Chem 47:967–972. doi:10.1002/jhet.399
Jadidi K, Ghahremanzadeh R, Bazgir A (2009) Efficient synthesis of spiro[chromeno[2,3-d]pyrimidine-5,3\(^{\prime }\)-indoline]-tetraones by a one-pot and three-component reaction. J Comb Chem 11:341–344. doi:10.1021/cc800167h
Deng J, Mo L-P, Zhao F-Y, Zhang Z-H, Liu S-X (2012) One-pot, three-component synthesis of a library of spirooxindole-pyrimidines catalyzed by magnetic nanoparticle supported dodecyl benzenesulfonic acid in aqueous media. ACS Comb Sci 14:335–341. doi:10.1021/co3000264
Moghaddam MM, Bazgir A, Mehdi AM, Ghahremanzadeh R (2012) Alum (KAl\(({\rm SO}_{4})_{2}\cdot 12{\rm H}_{2}{\rm O}\)) catalyzed multicomponent transformation: simple, efficient, and green route to synthesis of functionalized spiro[chromeno[2,3-d]pyrimidine-5,3\(^{\prime }\)-indoline]-tetraones in ionic liquid media. Chinese J Chem 30:709–714. doi:10.1002/cjoc.201280014
Ghahremanzadeh R, Amanpour T, Sayyafi M, Bazgir A (2010) One-pot, three-component synthesis of spironaphthopyrano[2,3-d]pyrimidine-5,3\(^{\prime }\)-indolines in water. J Heterocycl Chem 47:421–424. doi:10.1002/jhet.331
Tisseh ZN, Ahmadi F, Dabiri M, Khavasi HR, Bazgir A (2012) A novel organocatalytic multi-component reaction: an efficient synthesis of polysubstituted pyrano-fused spirooxin. Tetrahedron Lett 53:3603–3606. doi:10.1016/j.tetlet.2012.05.019
Bondensgaard K, Ankersen M, Thogersen H, Hansen BS, Wulff BS et al (2004) Recognition of privileged structures by g-protein coupled receptors. J Med Chem 47:888–899. doi:10.1021/jm0309452
Patchett AA, Nargund RP, Tata JR, Chen MH, Barakat KJ, Johnston DB, Cheng K, Chan WW, Butler B, Hickey G (1995) Design and biological activities of L-163,191 (MK-0677): a potent, orally active growth hormone secretagogue. PNAS 92:7001–7005. doi:10.1073/pnas.92.15.7001
Hickey G, Jacks T, Judith F, Taylor J, Schoen WR, Krupa D, Cunningham P, Clark J, Smith RG (1994) Efficacy and specificity of L-692,429, a novel nonpeptidyl growth hormone secretagogue, in beagles. Endocrinology 134:695–701. doi:10.1210/en.134.2.695
Sluder A, Shah S, Cassayre J, Clover R, Maienfisch P et al (2012) Spiroindolines identify the vesicular acetylcholine transporter as a novel target for insecticide action. PLoS One 7:e34712. doi:10.1371/journal.pone.0034712
Inoue S, Okada K, Tanino H, Hashizume K, Kakoi H (1994) Total synthesis of (\(\pm \))-surugatoxin. Heterocycl 50:2729–2752. doi:10.1016/S0040-4020(01)86989-1
Inoue S, Okada K, Tanino H, Kakoi H (1988) Synthesis of (+)-prosurugatoxin and ring transformation of prosurugatoxin into surugatoxin. Tetrahedron Lett 29:1547–1550. doi:10.1016/S0040-4039(00)80348-2
Seo JH, Liu P, Weinreb SM (2010) Evolution of a strategy for total synthesis of the marine fungal alkaloid \((\pm )\)-communesin F. J Org Chem 75:2667–2680. doi:10.1021/jo100339k
Lesma G, Landoni N, Sacchetti A, Silvani A (2010) The spiropiperidine-3,3\(^\prime \)-oxindoles caffold: a type II \({\beta }\)-turn peptideisostere. Tetrahedron 66:4474–4478. doi:10.1016/j.tet.2010.04.077
Han Y-Y, Han W-Y, Hou X, Zhang X-M, Yuan W-C (2012) \(\text{ FeCl }_{3}\)-catalyzed stereoselective construction of spirooxindole tetrahydroquinolines via tandem 1,5-hydride transfer/ring closure. Org Lett 14:4054–4057. doi:10.1021/ol301559k
Kiruthika SE, Lakshmi NV, Banu BR, Perumal PT (2011) A facile strategy for the one pot multicomponent synthesis of spiro dihydropyridines from amines and activated alkynes. Tetrahedron Lett 52:6508–6511. doi:10.1016/j.tetlet.2011.09.119
Alizadeh A, Mokhtari J (2011) Novel four-component route to the synthesis of spiro[indoline-3,4\(^{\prime }\)-pyridine]-3\(^{\prime }\)-carboxylate derivatives. Tetrahedron 67:3519–3523. doi:10.1016/j.tet.2011.03.032
Ghahremanzadeh R, Ahadi S, Shakibaei GI, Bazgir A (2010) Grindstone chemistry: one-pot synthesis of spiro[diindenopyridine-indoline]triones and spiro[acenaphthylene-diindenopyridine]triones. Tetrahedron Lett 51:499–502. doi:10.1016/j.tetlet.2009.11.041
Manpadi M et al (2007) Three-component synthesis and anticancer evaluation of polycyclic indenopyridines lead to the discovery of a novel indenoheterocycle with potent apoptosis inducing properties. Org Biomol Chem 5:3865–3872. doi:10.1039/B713820B
Ahadi S, Ghahremanzadeh R, Mirzaei P, Bazgir A (2009) Synthesis of spiro[benzopyrazolonaphthyridine-indoline]-diones and spiro[chromenopyrazolopyridine-indoline]-diones by one-pot, three-component methods in water. Tetrahedron 65:9316–9321. doi:10.1016/j.tet.2009.09.009
Quiroga J, Portillo S, Pérez A, Gálvez J, Abonia R, Insuasty B (2011) An efficient synthesis of pyrazolo[3,4-b]pyridine-4-spiroindolinones by a three-component reaction of 5-aminopyrazoles, isatin, and cyclic \({\beta }\)-diketones. Tetrahedron Lett 52:2664–2666. doi:10.1016/j.tetlet.2011.03.067
Ghahremanzadeh R, Sayyafi M, Ahadi S, Bazgir A (2009) Novel one-pot, three-component synthesis of spiro[indoline-pyrazolo[4\(^{\prime }\),3\(^{\prime }\):5,6]pyrido[2,3-d]pyrimidine]trione library. J Comb Chem 11:393–396. doi:10.1021/cc8001958
Shakibaei GI, Feiz A, Bazgir A (2011) A simple and catalyst-free three-component method for the synthesis of spiro[indenopyrazolopyridine indoline]diones and spiro[indenopyridopyrimidine indoline]triones. Comptes Rendus Chimie 14:556–562. doi:10.1016/j.crci.2010.10.001
Jadidi K, Ghahremanzadeh R, Bazgir A (2009) Spirooxindoles: reaction of 2,6-diaminopyrimidin-4(3H)-one and isatins. Tetrahedron 65:2005–2009. doi:10.1016/j.tet.2009.01.013
Shakibaei GI, Feiz A, Khavasi HR, Soorki AA, Bazgir A (2011) Simple three-component method for the synthesis of spiroindeno[1,2-b]pyrido[2,3-d]pyrimidine-5,3\(^\prime \)-indolines. ACS Comb Sci 13:96–99. doi:10.1021/co1000053
Rahmati A, Khalesi Z (2012) A one-pot, three-component synthesis of spiro[indolineisoxazolo[4\(^{\prime }\),3\(^{\prime }\):5,6]pyrido[2,3-d]pyrimidine]triones in water. Tetrahedron 68:8472–8479. doi:10.1016/j.tet.2012.07.073
Venkatesan H, Davis MC, Altas Y, Snyder JP, Liotta DC (2001) Total synthesis of SR 121463 A, a highly potent and selective vasopressin \(\text{ V }_{2}\) receptor antagonist. J Org Chem 66:3653–3661. doi:10.1021/jo0004658
Liu J-J, Zhang Z (2008) Spiroindolinone derivatives (Hoffmann-La Roche AG). PCT Int Appl. WO2008(055812)
Sconhaber J, Mueller ThJJ (2011) Luminescent bichromophoric spiroindolones—synthesis and electronic properties. Org Biomol Chem 9:6196–6199. doi:10.1039/c1ob05703k
Beccalli EM, Clerici F, Gelmi ML (1999) 6-Chloro-spiroeyelohexenindol-2-ones: an unusual ring transformation to ethyl 2-(cyelohexa-1,4-dlenyl)phenylearbamates. Tetrahedron 55:8579–8586. doi:10.1016/S0040-4020(99)00475-5
Beccalli EM, Clerici F, Gelmi ML (2003) A new synthetic procedure to spiro[cyclohexane-1,3\(^{\prime }\)-indoline]-2\(^{\prime }\),4-diones. Tetrahedron 59:4615–4622. doi:10.1016/S0040-4020(03)00627-6
Ashimori’ A, Overman LE (1992) Catalytic asymmetric synthesis of quarternary carbon centers. Palladium-catalyzed formation of either enantiomer of spirooxindoles and related spirocyclics using a single enantiomer of a chiral diphosphine ligand. J Org Chem 57(4571–4572):4671. doi:10.1021/jo00043a005
Jia ZJ, Jiang H, Li JL, Gschwend B, Li QZ, Yin X, Grouleff J, Chen YC, Jørgensen KA (2011) Trienamines in asymmetric organocatalysis: Diels-Alder and tandem reactions. J Am Chem Soc 133:5053–5061. doi:10.1021/ja1112194
Tan B, Hernández-Torres G, Barbas CF (2011) Highly efficient hydrogen-bonding catalysis of the Diels-Alder reaction of 3-vinylindoles and methyleneindolinones provides carbazolespirooxindole skeletons. J Am Chem Soc 133:12354–12357. doi:10.1021/ja203812h
Wei Q, Gong L-Z (2010) Organocatalytic asymmetric formal [4+2] cycloaddition for the synthesis of spiro[4-cyclohexanone-1,3\(^{\prime }\)-oxindoline] derivatives in high optical purity. Org Lett 12:1008–1011. doi:10.1021/ol100020v
Li Y, Su X, Zhou W, Li W, Zhang J (2015) Amino-acid derived phosphine-catalyzed enantioselective 1,4- dipolar spiroannulation of cyclobutenones and isatylidenemalononitrile. Chem Eur J 21:1–6. doi:10.1002/chem.201406475
Richmond E, Duguet N, Slawin AMZ, Lébl T, Smith AD (2012) Asymmetric pericyclic cascade approach to spirocyclic oxindoles. Org Lett 14:2762–2765. doi:10.1021/ol300982f
Bencivenni G, Wu LY, Mazzanti A, Giannichi B, Pesciaioli F, Song MP, Bartoli G, Melchiorre P (2009) Targeting structural and stereochemical complexity by organocascade catalysis: construction of spirocyclic oxindoles having multiple stereocenters. Angew Chem Int Ed 48:7200–7203. doi:10.1002/anie.200903192
Jiang K, Jia Z-J, Yin X, Wu L, Chen Y-C (2010) Asymmetric quadruple aminocatalytic domino reactions to fused carbocycles incorporating a spirooxindole motif. Org Lett 12:2766–2769. doi:10.1021/ol100857s
Jiang K, Jia Z-J, Chen S, Li Wu, Chen Y-C (2010) Organocatalytic tandem reaction to construct six-membered spirocyclic oxindoles with multiple chiral centres through a formal [2+2+2] annulation. Chem Eur J 16:2852–2856. doi:10.1002/chem.200903009
Ghosh AK, Zhou B (2013) Enantioselective synthesis of spiro[cyclohexane-1,3\(^\prime \)-indolin]-2\(^\prime \)-ones containing multiple stereocenters via organocatalytic Michael/aldol cascade reactions. Tetrahedron Lett 54:2311–2314. doi:10.1016/j.tetlet.2013.02.030
Companyo X, Zea A, Alba A-NR, Mazzanti A, Moyano A, Rios R (2010) Organocatalytic synthesis of spiro compounds via a cascade Michael-Michael-aldol reaction. Chem Commun 46:6953–6955. doi:10.1039/C0CC01522A
Lan YB, Zhao H, Liu ZM, Liu GG, Tao JC, Wang XW (2011) Chiral counteranion synergistic organocatalysis under high temperature: efficient construction of optically pure spiro[cyclohexanone-oxindole] backbone. Org Lett 13:4866–4869. doi:10.1021/ol201943g
Sun QS, Chen X-Y, Zhu H, Lin H, Sun X-W, Lin G-Q (2015) Asymmetric synthesis of poly-substituted spirocyclohexane oxindole via a squaramide catalyzed cascade Michael-Michael-aldol sequence. Org Chem Front 2:110–113. doi:10.1039/C4QO00299G
Basavaiah D, Reddy KR (2007) Simple and one-pot protocol for synthesis of indene-spiro-oxindoles involving tandem prins and friedel-crafts reactions. Org Lett 9:57–60. doi:10.1021/ol062561m
Gorokhovik I, Neuville L, Zhu J (2011) Trifluoroacetic acid-promoted synthesis of 3-hydroxy, 3-amino and spirooxindoles from \({\alpha }\)-keto-N-anilides. Org Lett 13:5536–5539. doi:10.1021/ol202263a
Bond RF, Boeyens JCA, Holzapfel CW, Steyn PS (1979) Cyclopiamines A and B, novel oxindole metabolites of Penicillium cyclopium westling. J Chem Soc, Perkin Trans 1:1751–1761. doi:10.1039/P19790001751
Mugishima T, Tsuda M, Kasai Y, Ishiyama H, Fukushi E, Kawabata J, Watanabe M, Akao K, Kobayashi J (2005) Absolute stereochemistry of citrinadins A and B from marine-derived fungus. J Org Chem 70:9430–9435. doi:10.1021/jo051499o
Pettersson M, Knueppel D, Martin SF (2007) Concise, stereoselective approach to the spirooxindole ring system of citrinadin A. Org Lett 9:4623–4626. doi:10.1021/ol702132v
Bian Z, Marvin CC, Martin SF (2013) Enantioselective total synthesis of (-)-citrinadin A and revision of its stereochemical structure. J Am Chem Soc 135:10886–10889. doi:10.1021/ja405547f
Guerrero CA, Sorensen EJ (2011) Concise, stereocontrolled synthesis of the citrinadin B core architecture. Org Lett 13:5164–5167. doi:10.1021/ol2020362
Kong K, Enquist JA Jr, McCallum ME, Smith GM, Matsumaru T, Menhaji-Klotz E, Wood JL (2013) An enantioselective total synthesis and stereochemical revision of (+)-citrinadin B. J Am Chem Soc 135:10890–10893. doi:10.1021/ja405548b
Li X, Li YM, Peng FZ, Wu ST, Li ZQ, Sun ZW, Zhang HB, Shao ZH (2011) Highly enantioselective one-pot synthesis of spirocyclopentaneoxindoles containing the oxime group by organocatalyzed Michael addition/ISOC/fragmentation sequence. Org Lett 13:6160–6163. doi:10.1021/ol2024955
Albertshofer K, Tan B, Barbas CF (2012) Assembly of spirooxindole derivatives containing four consecutive stereocenters via organocatalytic Michael-Henry cascade reactions. Org Lett 14:1834–1837. doi:10.1021/ol300441z
Albertshofer K, Anderson KE, Barbas CF (2012) Assembly of spirooxindole derivatives via organocatalytic iminium-enamine cascade reactions. Org Lett 14:5968–5971. doi:10.1021/ol302876c
Jiang K, Tiwari B, Chi YR (2012) Access to spirocyclic oxindoles via N-heterocyclic carbene-catalyzed reactions of enals and oxindole-derived \({\alpha },{\beta }\)-unsaturated imines. Org Lett 14:2382–2385. doi:10.1021/ol3008028
Sun W, Zhu G, Wu C, Hong L, Wang R (2012) An organocatalytic cascade strategy for the enantioselective construction of spirocyclopentane bioxindoles containing three contiguous stereocenters and two spiro quaternary centers. Chem Eur J 18:6737–6741. doi:10.1002/chem.201200478
Sun W, Zhu G, Wu C, Hong L, Wang R (2012) “Organo-metal” synergistic catalysis: the 1+1\(>\)2 effect for the construction of spirocyclopentene oxindoles. Chem Eur J 18:13959–13963. doi:10.1002/chem.201201976
Tian X, Melchiorre P (2013) Control of remote stereochemistry in the synthesis of spirocyclic oxindoles: vinylogous organocascade catalysis. Angew Chem Int Ed 52:1–5. doi:10.1002/anie.201301017
Ding L-Z, Zhong T-S, Wu T, Wang Y-M (2014) Highly enantioselective construction of spirocyclopentaneoxindoles containing four consecutive stereocenters through an organocatalytic iminium–enamine cascade reaction. Eur J Org Chem 24:5139–5143. doi:10.1002/ejoc.201402687
Trost BM, Cramer N, Silverman SM (2007) Enantioselective construction of spirocyclic oxindolic cyclopentanes by palladium-catalyzed trimethylenemethane-[3+2]-cycloaddition. J Am Chem Soc 129:12396–12397. doi:10.1021/ja075335w
Voituriez A, Pinto N, Neel M, Retailleau P, Marinetti A (2010) An organocatalytic [3+2] cyclisation strategy for the highly enantioselective synthesis of spirooxindoles. Chem Eur J 16:12541–12544. doi:10.1002/chem.201001791
Tan B, Candeias NR, Barbas CF (2011) Core-structure-motivated design of a phosphine-catalyzed [3+2] cycloaddition reaction: enantioselective syntheses of spirocyclopenteneoxindoles. J Am Chem Soc 133:4672–4675. doi:10.1021/ja110147w
Peng J, Huang X, Jiang L, Cui H-L, Chen Y-C (2011) Tertiary amine-catalyzed chemoselective and asymmetric [3+2] annulation of Morita-Baylis-Hillman carbonates of isatins with propargyl sulfones. Org Lett 13:4584–4587. doi:10.1021/ol201776h
Wang Y, Liu L, Zhang T, Zhong N-J, Wang D, Chen Y-J (2012) Diastereo- and enantioselective [3+2] cycloaddition reaction of Morita-Baylis-Hillman carbonates of isatins with n-phenylmaleimide catalyzed by Me-Duphos. J Org Chem 77:4143–4147. doi:10.1021/jo3002535
Jiang T, Kuhen KL, Wolff K, Yin H, Bieza K, Caldwell J, Bursulaya B, Wu TYH, He Y (2006) Design, synthesis and biological evaluations of novel oxindoles as HIV-1 non-nucleoside reverse transcriptase inhibitors. Part I. Bioorg Med Chem Lett 16:2105–2108. doi:10.1016/j.bmcl.2006.01.073
Jiang T, Kuhen KL, Wolff K, Yin H, Bieza K, Caldwell J, Bursulaya B, Wu TYH, Tuntland T, Zhang K, Karanewsky D, He Y (2006) Design, synthesis and biological evaluations of novel oxindoles as HIV-1 non-nucleoside reverse transcriptase inhibitors. Part II. Bioorg Med Chem Lett 16:2109–2112. doi:10.1016/j.bmcl.2006.01.066
Dou X, Lu Y (2012) Diastereodivergent synthesis of 3-spirocyclopropyl-2-oxindoles through direct enantioselective cyclopropanation of oxindoles. Chem Eur J 18:8315–8319. doi:10.1002/chem.201200655
Shaabanzadeh M, Khabari F (2010) One-pot synthesis of new spiro[cyclopropane-1,3\(^{\prime }\)-[3H]indol]-2\(^{\prime }\)(1\(^{\prime }\)H)-ones from 3-phenacylideneoxindoles. J Heterocycl Chem 47:949–953. doi:10.1002/jhet.394
Kumari G, Nutan Modi M, Gupta SK, Singh RK (2011) Rhodium(II) acetate-catalyzed stereoselective synthesis, SAR and anti-HIV activity of novel oxindoles bearing cyclopropane ring. Eur J Med Chem 46:1181–1188. doi:10.1016/j.ejmech.2011.01.037
Muthusamy S, Azhagan D, Gnanaprakasam B, Suresh E (2010) Diastereoselective synthesis of strained spiro-cyclopropanooxindoles from cyclic diazoamides. Tetrahedron Lett 51:5662–5665. doi:10.1016/j.tetlet.2010.07.159
Muthusamy S, Ramkumar R (2014) Solvent- and transition metal-free synthesis of spiro[cyclopropane-1,3- oxindoles] from cyclic diazoamides. Tetrahedron Lett 55:6389–6393. doi:10.1016/j.tetlet.2014.09.086
Oseka M, Noole A, Zari S, Öeren M, Järving I, Lopp M, Kanger T (2014) Asymmetric diastereoselective synthesis of spirocyclopropane derivatives of oxindole. Eur J Org Chem 17:3599–3606. doi:10.1002/ejoc.201402061
Pedras MSC, Okanga FI, Zaharia IL, Khan AQ (2000) Phytoalexins from crucifers: synthesis, biosynthesis, and Biotransformation. Phytochem 53:161–176. doi:10.1016/S0031-9422(99)00494-X
Kutschy P, Salayová A, Curillová Z, Kozár T, Mezencev R, Mojzis J, Pilátová M, Balentová E, Pazdera P, Sabol M, Zburová M (2009) 2-(Substituted phenyl)amino analogs of 1-methoxyspirobrassinol methyl ether: synthesis and anticancer activity. Bioorg Med Chem 17:3698–3712. doi:10.1016/j.bmc.2009.03.064
Kutschy P, Suchý M, Monde K, Harada N, Marušková R, Čurillová Z, Dzurilla M, Miklošová M, Mezencev R, Mojžiš J (2002) Spirocyclization strategy toward indole phytoalexins. The first synthesis of \((\pm )\)-1-methoxyspirobrassinin, \((\pm )\)-1-methoxyspirobrassinol, and \((\pm )\)-1-methoxyspirobrassinol methyl ether. Tetrahedron Lett 43:9489–9492. doi:10.1016/S0040-4039(02)02452-8
Zhang Y, Li ZJ, Xu HS, Zhang Y, Wang W (2011) Organocatalytic asymmetric Henry reaction of isatins: highly enantioselective synthesis of 3-hydroxy-2-oxindoles. RSC Adv 1:389–392. doi:10.1039/C1RA00477H
Chen W-B, Wu Z-J, Hu J, Cun J-F, Zhang X-M, Yuan W-C (2011) Organocatalytic direct asymmetric aldol reactions of 3-isothiocyanato oxindoles to ketones: stereocontrolled synthesis of spirooxindoles bearing highly congested contiguous tetrasubstituted stereocenters. Org Lett 13:2472–2475. doi:10.1021/ol200724q
Han Y-Y, Chen W-B, Han W-Y, Wu Z-J, Zhang X-M, Yuan W-C (2012) Highly efficient and stereoselective construction of dispiro-[oxazolidine-2-thione]bisoxindoles and dispiro[imidazolidine-2-thione]bisoxindoles. Org Lett 14:490–493. doi:10.1021/ol203081x
Jiang X, Cao Y, Wang Y, Liu L, Shen F, Wang R (2010) A unique approach to the concise synthesis of highly optically active spirooxazolines and the discovery of a more potent oxindole-type phytoalexin analogue. J Am Chem Soc 132:15328–15333. doi:10.1021/ja106349m
Badillo JJ, Arevalo GE, Fettinger JC, Franz AK (2011) Titanium-catalyzed stereoselective synthesis of spirooxindole oxazolines. Org Lett 13:418–421. doi:10.1021/ol1027305
Ueda T, Inada M, Okamoto I, Morita N, Tamura O (2008) Synthesis of maremycins a and d1 via cycloaddition of a nitrone with (e)-3-ethylidene-1-methylindolin-2-one. Org Lett 10:2043–2046. doi:10.1021/ol800515w
Singh A, Roth GP (2011) A [3+2] dipolar cycloaddition route to 3-hydroxy-3-alkyl oxindoles: an approach to pyrrolidinoindoline alkaloids. Org Lett 13:2118–2121. doi:10.1021/ol200547m
Bouhfid R, Joly N, Essassi EM, Lequart V, Massoui M, Martin P (2011) Synthesis of new spiro[1,4,2-dioxazole-5,3\(^{\prime }\)-indolin]-2\(^{\prime }\)-one by 1,3-dipolar cycloaddition. Synth Commun 41:2096–2102. doi:10.1080/00397911.2010.497595
Ribeiro CJA, Kumar SP, Moreira R, Santos MMM (2012) Efficient synthesis of spiroisoxazoline oxindoles. Tetrahedron Lett 53:281–284. doi:10.1016/j.tetlet.2011.10.139
Gomez-Monterrey I, Bertamino A, Porta A, Carotenuto A, Musella S, Aquino C, Granata I, Sala M, Brancaccio D, Picone D, Ercole C, Stiuso P, Campiglia P, Grieco P, Ianelli P, Maresca B, Novellino E (2010) Identification of the spiro(oxindole-3,3’-thiazolidine)-based derivatives as potential p53 activity modulators. J Med Chem 53(8319–8329):8319. doi:10.1021/jm100838z
Vintonyak VV, Warburg K, Kruse H, Grimme S, Hubel K, Rauh D, Waldmann H (2010) Identification of thiazolidinones spiro-fused to indolin-2-ones as potent and selective inhibitors of the mycobacterium tuberculosis protein tyrosine phosphatase B. Angew Chem Int Ed 49:5902–5905. doi:10.1002/anie.201002138
Kaminskyy D, Khyluk D, Vasylenko O, Zaprutko L, Lesyk R (2011) A facile synthesis and anticancer activity evaluation of spiro[thiazolidinone-isatin] conjugates. Sci Pharm 79:763–777. doi:10.3797/scipharm.1109-14
Chen H, Shi D (2011) Efficient one-pot synthesis of spiro[indoline-3,40-pyrazolo[3,4-e][1,4]thiazepine]dione via three-component reaction. Tetrahedron 67:5686–5692. doi:10.1016/j.tet.2011.05.069
Cao Y, Shen FF, Zhang FT, Wang R (2012) Catalytic asymmetric michael addition/cyclization of isothiocyanato oxindoles: highly efficient and versatile approach for the synthesis of 3,2\(^{\prime }\)-pyrrolidinyl mono- and bi-spirooxindole frameworks. Chem Eur J 19:1184–1188. doi:10.1002/chem.201204114
Wu H, Zhang LL, Tian ZQ, Huang YD, Wang YM (2012) Highly efficient enantioselective construct hion of bispirooxindoles containing three stereocenters through an organocatalytic cascade michael-cyclization reaction. Chem Eur J 15:1246–1249. doi:10.1002/chem.201203221
Curillova’ Z, Kutschy P, Budovska M, Nakahashib A, Mondeb K (2007) Stereoselective synthesis of (R)-(+)-1-methoxyspirobrassinin, (2R,3R)-(-)-1-methoxyspirobrassinol methyl ether and their enantiomers or diastereoisomers. Tetrahedron Lett 48:8200–8204. doi:10.1016/j.tetlet.2007.09.080
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pavlovska, T.L., Redkin, R.G., Lipson, V.V. et al. Molecular diversity of spirooxindoles. Synthesis and biological activity. Mol Divers 20, 299–344 (2016). https://doi.org/10.1007/s11030-015-9629-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-015-9629-8