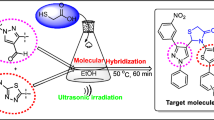
Abstract
An environmentally benign, simple, efficient, and convenient route is described for the synthesis of novel pyrazolo[1,5-a]pyrimidine derivatives under ultrasound irradiation. Condensation of aminopyrazole 5 with formylated active proton compounds (6, 8, E–G, 12, and 15) furnished pyrazolopyrimidine (7, 9, 10, 13, and 16) in high-to-excellent yields. In comparison with conventional methods, ultrasound irradiation offers several advantages, such as shorter reaction time, higher yields, milder conditions, and environmental friendliness. The reaction is clean with excellent yields and reduces the use of solvents. X-ray crystallographic study of compound 7c confirmed the regioselectivity of the reaction. The antibacterial profile of the newly synthesized compounds was evaluated by cup and saucer method.








Similar content being viewed by others
References
Zask A, Verheijen JC, Curran K, Kaplan J, Richard DJ, Nowak P, Malwitz DJ, Brooijmans N, Bard J, Svenson K, Lucas J, Toral-Barza L, Zhang WG, Hollander I, Gibbons JJ, Abraham RT, Ayral-Kaloustian S, Mansour TS, Yu K (2009) ATP-competitive inhibitors of the mammalian target of rapamycin: design and synthesis of highly potent and selective pyrazolopyrimidines. J Med Chem 52:5013–5016. doi:10.1021/jm900851f
Curran K, Verheijen JC, Kaplan J, Richard DJ, Toral-Barza L, Hollander I, Lucas J, Ayral-Kaloustian S, Yu K, Zask A (2010) Pyrazolopyrimidines as highly potent and selective, ATP-competitive inhibitors of the mammalian target of rapamycin (mTOR): optimization of the 1-substituent. Bioorg Med Chem Lett 20:1440–1444. doi:10.1016/j.bmcl.2009.12.086
Stefano A, Anna A, Maurizio B, Alessandra T, Francisco O, Francesco O, Silvia S, Chiara B, Matilde Y (2010) Hit identification and biological evaluation of anticancer pyrazolopyrimidines endowed with anti-inflammatory activity. Chem Med Chem 5:1242–1246. doi:10.1002/cmdc.201000165
Huang S, Lin R, Yu Y, Lu Y, Conolly P, Chiu G, Li S, Emanuel S, Middleton S (2007) Synthesis of 3-(1H-benzimidazol-2-yl)-5-isoquinolin-4-ylpyrazolo[1,2-b]pyridine, a potent cyclin dependent kinase 1 (CDK1) inhibitor. Bioorg Med Chem Lett 17:1243–1245. doi:10.1016/j.bmcl.2006.12.031
Mukaiyama H, Nishimura T, Kobayashi S, Komatsu Y (2008) Novel pyrazolo[1,5-\(a\)]pyrimidines as c-Src kinase inhibitors that reduce \(I_{{\rm Kr}}\) channel blockade. Bioorg Med Chem 16:909–921. doi:10.1016/j.bmc.2007.10.068
Rao VVVNSR, Lingaiah BPV, Reddy GV, Ezikiel G, Yadla R, Rao PS (2006) Facile synthesis of fluorinated 2-aryl-5,7-bisalkyl pyrazolopyrimidines from arylalkynenitriles. Arkivoc xii:51–57. doi:10.3998/ark.5550190.0007.c06
Li M, Guo W-S, Wen L-R, Qu B (2006) Synthesis, structure and biological activities of 2-methylthio-3-cyano-7-(4-methoxyphenyl)-pyrazolo[1,5-\(a\)]pyrimidine. Chin J Struct Chem 25:108–112
Ghorab MM, Ismail ZH, Abdel-Gawad SM, Aziem AA (2004) Antimicrobial activity of amino acid, imidazole, and sulfonamide derivatives of pyrazolo[3,4-\(d\)]pyrimidine. Heteroat Chem 15:57–62. doi:10.1002/hc.10212
Ahmed OM, Mohamed MA, Ahmed RR, Ahmed SA (2009) Synthesis and anti-tumor activities of some new pyridines and pyrazolo[1,5-a]pyrimidines. Eur J Med Chem 44:3519–3523. doi:10.1016/j.ejmech.2009.03.042
El-Enanya MM, Kamelb MM, Khalilb OM, El-Nassanb HB (2011) Synthesis and antitumor activity of novel pyrazolo[1,5-a]pyrimidine derivatives. Eur J Chem 2:331–336. doi:10.5155/eurjchem.2.3.331-336.319
Wang YD, Honores E, Wu B, Johnson S, Powell D, Miranda M, McGinnis JP, Discafani C, Rabindran SK, Cheng W, Krishnamurthy G (2009) Synthesis, SAR study and biological evaluation of novel pyrazolo[1,5-a]pyrimidin-7-yl phenyl amides as anti-proliferative agents. Bioorg Med Chem 17:2091–2100. doi:10.1016/j.bmc.2008.12.046
Avila JL, Polegre MA, Avila AR, Robins K (1986) Action of pyrazolopyrimidine derivatives on American Leishmania spp. promastigotes. Comp Biochem Physiol 83C:285–289. doi:10.1016/0742-8413(86)90124-6
Al-Adiwish WM, Tahir MIM, Siti-Noor-Adnalizawati A, Hashim SF, Ibrahim N, Yaacob WA (2013) Synthesis, antibacterial activity and cytotoxicity of new fused pyrazolo[1,5-a]pyrimidine and pyrazolo[5,1-c][1,2,4]triazine derivatives from new 5-aminopyrazoles. E J Med Chem 64:464–476. doi:10.1016/j.ejmech.2013.04.029
Fraley ME, Hoffman WF, Rubino RS, Hungate RW, Tebben AJ, Rutledge RZ, McFall RC, Huckle WR, Kendall RL, Coll KE, Thomas KA (2002) Synthesis and initial SAR studies of 3,6-disubstituted pyrazolo[1,5-a]pyrimidines: a new class of KDR kinase inhibitors. Bioorg Med Chem Lett 12:2767–2770. doi:10.1016/S0960-894X(02)00525-5
Fraley ME, Rubino RS, Hoffman WF, Hambaugh SR, Arrington KL, Hungate RW, Bilodeau MT, Tebben AJ, Rutledge RZ, Kendall RL, McFall RC, Huckle WR, Coll KE, Thomas KA (2003) Optimization of a pyrazolo[1,5-a]pyrimidine class of KDR kinase inhibitors: improvements in physical properties enhance cellular activity and pharmacokinetics. Bioorg Med Chem Lett 12:3537–3541. doi:10.1016/S0960-894X(02)00827-2
Compton DR, Carlson KE, Katzenellenbogen JA (2004) Pyrazolo[1,5-a]pyrimidines as estrogen receptor ligands: defining the orientation of a novel heterocyclic core. Bioorg Med Chem Lett 14:5681–5684. doi:10.1016/j.bmcl.2004.08.046
Mokhtara M, Saleha TS, Basahel SN (2012) Mg-Al hydrotalcites as efficient catalysts for aza-Michael addition reaction: a green protocol. J Mol Catal A 353–354:122–131. doi:10.1016/j.molcata.2011.11.015
Ahmetaj S, Velikanje N, Grošelj U, Šterbal I, Prek B, Golobič A, Kočar D, Dahmann G, Stanovnik B, Svete J (2013) Parallel synthesis of 7-heteroaryl-pyrazolo[1,5-a]pyrimidine-3-carboxamides. Mol Divers 17:731–743. doi:10.1007/s11030-013-9469-3
Zhao P-L, Wang L, Zhu X-L, Huang X, Zhan C-G, Wu J-W, Yang G-F (2010) Subnanomolar inhibitor of cytochrome bc1 complex designed by optimizing interaction with conformationally flexible residues. J Am Chem Soc 132:185–194. doi:10.1021/ja905756c
Zhao P-L, Wang L, Zhang M-Z, Liu Z-M, Huang W, Yang G-F (2008) Synthesis, fungicidal, and insecticidal activities of \(\beta \)-methoxyacrylate-containing N-acetyl pyrazoline derivatives. J Agric Food Chem 56:10767–10773. doi:10.1021/jf802343p
Zhibing W, Deyu H, Jiqing K, Hua C, Shixi W, Wei X (2012) Synthesis and antifungal activity of \(N\)-(substituted pyridinyl)-1-methyl(phenyl)-3-(trifluoromethyl)-1\(H\)-pyrazole-4-carboxamide derivatives. Molecules 17:14205–14218. doi:10.3390/molecules171214205
Patnaik S, Zheng W, Choi JH, Motabar O, Southall N, Westbroek W, Lea WA, Velayati A, Goldin E, Sidransky E, Leister W, Marugan JJ (2012) Discovery, structure-activity relationship, and biological evaluation of noninhibitory small molecule chaperones of glucocerebrosidase. J Med Chem 55:5734–5748. doi:10.1021/jm300063b
Elnagdi MH, Elmoghayar MRH, Elgemeie GEH (1987) Chemistry of pyrazolopyimidines. Adv Heterocycl Chem 41:319–376. doi:10.1016/S0065-2725(08)60164-6
Dwyer MP, Paruch K, Labroli M, Alvarez C, Keertikar KM, Poker C, Rossman R, Fischmann TO, Duca JS, Madison V, Parry D, Davis N, Seghezzi W, Wiswell D, Guzi TJ (2011) Discovery of pyrazolo[1,5-a]pyrimidine-based CHK1 inhibitors: a template-based approach-part 1. Bioorg Med Chem Lett 21:467–470. doi:10.1016/j.bmcl.2010.10.113
Shiota T, Yamomori T, Sakai K, Kiyokawa M, Honma T, Ogawa M, Hayashi K, Ishizuka N, Matsumura KI, Hara M, Fujimoto M, Kawabata T, Nakajima S (1999) Synthesis and structure-activity relationship of a new series of potent angiotensin II receptor antagonists: pyrazolo[1, 5-\(\alpha \)]pyrimidine derivatives. Chem Pharm Bull 47:928–938. doi:10.1248/cpb.47.928
Martins MAP, Scapin E, Frizzo CP, Rosa FA, Bonacorso HG, Zanatta N (2009) 2-Methyl-7-substituted pyrazolo[1,5-\(a\)]pyrimidines: highly regioselective synthesis and bromination. J Braz Chem Soc 20:205–213. doi:10.1590/S0103-50532009000200003
Al-Enezi A, Al-Saleh B, Elnagdi MH (1997) Studies with heteroatomic amines: the reaction of some heteroatomic amines with 1-substituted 3-dimethylaminopropanones, enaminones and cinnamonitriles. J Chem Res (S):4-5. doi:10.1039/A602931K
Dusza JP, Tomcufcik AS, Albright JD (1985) [7-(3-disubstituted amino)phenyl]pyrazolo[1,5-a]pyrimidines. Patent US 4626538
Al-Mousawi SM, Moustafa MS, Elnagdi MH (2010) Polyfunctional heteroaromatics: a route to dicyanomethylene thiazoles based on the reaction of \(\alpha \)-thiocyanatoketones with malononitrile. Arkivoc ii:224–232.doi:10.3998/ark.5550190.0011.217
Behbehani H, Ibrahim HM, Makhseed S (2010) Synthesis of 7-substituted pyrazolo[1,5-a]pyrimidine-3-carboxamides as potential non benzodiazepine hypnotics. Arkivoc ii:267–282. doi:10.3998/ark.5550190.0011.222
Gommermann N, Buehlmayer P, Matt A, Breitenstein W, Masuya K, Pirard B, Furet P, Cowan-Jacob SW, Weckbecker G (2010) New pyrazolo[1,5a]pyrimidines as orally active inhibitors of Lck. Bioorg Med Chem Lett 20:3628–3631. doi:10.1016/j.bmcl.2010.04.112
Frey RR, Curtin ML, Albert DH, Glaser KB, Pease LJ, Soni NB, Bouska JJ, Reuter D, Stewart K, Marcotte P, Bukofzer G, Li J, Davidsen SK, Michaelides MR (2008) 7-Aminopyrazolo[1,5-a]pyrimidines as potent multitargeted receptor tyrosine kinase inhibitors. J Med Chem 51:3777–3787. doi:10.1021/jm701397k
Elmaati TMA (2002) Reactions with heterocyclic amidines: new routes for the synthesis of novel azolo[1,5-a]pyrimidine, benzo[4,5]imidazo[1,2-a]pyrimidine, some pyridine, pyran and pyrazole derivatives containing the antipyrine moiety. Acta Chim Slov 49:721–732
Gregg BT, Tymoshenko DO, Razzano DA, Johnson MR (2007) Pyrazolo[1,5-\(a\)]pyrimidines. Identification of the privileged structure and combinatorial synthesis of 3-(hetero)arylpyrazolo[1,5-\(a\)]pyrimidine-6-carboxamides. J Comb Chem 9:507–512. doi:10.1021/cc0700039
Sadek KU, Mekheimer RA, Mohamed TM, Moustafa MS, Elnagdi MH (2012) Regioselectivity in the multicomponent reaction of 5-aminopyrazoles, cyclic 1,3-diketones and dimethylformamide dimethylacetal under controlled microwave heating. Beilstein J Org Chem 8:18–24. doi:10.3762/bjoc.8.3
El-Kateb AA, El-Rahman ANM, Saleh TS, Zeid IF, Mady MF (2012) Microwave-assisted synthesis of novel pyrazole, pyrimidine and pyrazolo[1,5-\(a\)]pyrimidines containing aryl sulfone moiety. Life Sci J 9:711–718
Quiroga J, Portilla J, Cruz S, Abonia R, Insuasty B, Nogueras M, Cobo J, Hursthouse M (2008) Solvent free synthesis of fused pyrazolo[1,5-\(a\)]pyrimidines by reaction of 5-mino-1H-pyrazoles and \(\beta \)-triketones. Open Org J 2:92–99. doi:10.2174/1874095200801020092
Zhang X, Song Y, Gao L, Guoa X, Fan X (2014) Highly facile and regio-selective synthesis of pyrazolo[1,5-a]pyrimidines via reactions of 1,2-allenic ketones with aminopyrazoles. Org Biomol Chem 12:2099–2107. doi:10.1039/C3OB42445F
Buriol L, Munchen TS, Frizzo CP, Marzari MRB, Zanatta N, Bonacorso HG, Martins MAP (2013) Resourceful synthesis of pyrazolo[1,5-a]pyrimidines under ultrasound irradiation. Ultrason Sonochem 20:1139–1143. doi:10.1016/j.ultsonch.2013.02.006
Bretanha LC, Teixeira VE, Ritter M, Siqueira GM, Cunico W, Pereira CMP, Freitag RA (2011) Ultrasound promoted synthesis of 3-trichloromethyl-5-alkyl(aryl)-1,2,4-oxadiazoles. Ultrason Sonochem 18:704–707. doi:10.1016/j.ultsonch.2013.02.006
Duarte A, Cucino W, Pereira CMP, Flores AFC, Freitag RA, Siqueira GM (2010) Ultrasound promoted synthesis of thioesters from 2-mercaptobenzoxa(thia)zoles. Ultrason Sonochem 17:281–283. doi:10.1016/j.ultsonch.2009.08.004
Kalita U, Kaping S, Nellanant J, Helissey P, Vishwakarma JN (2014) A facile ultrasound-assisted regioselective synthetic strategy of pyrazolo[1,5-a]pyrimidines mediated by \(\text{ KHSO }_{4}\) in aqueous media. Heterocycl Lett 4:137–145
Devi AS, Kaping S, Vishwakarma JN (2015) A facile environment-friendly one-pot two-step regioselective synthetic strategy for 3,7-diarylpyrazolo[1,5-a]pyrimidines related to zaleplon and 3,6-diarylpyrazolo[1,5-a]pyrimidine-7-amines assisted by \(\text{ KHSO }_{4}\) in aqueous media. Mol Divers. doi:10.1007/s11030-015-9606-2
Fadda AA, Bondock S, Rabie R, Etman HA (2008) Cyanoacetamide derivatives as synthons in heterocyclic synthesis. Turk J Chem 32:259–286
Chanda K, Dutta MC, Karim E, Vishwakarma JN (2004) An efficient, microwave assisted solvent-free synthesis of polarized enamines. J Indian Chem Soc 81:791–793
Chanda K, Dutta MC, Vishwakarma JN (2004) An efficient microwave assisted solvent-free general routes to cyclic enaminones. Ind J Chem 43B:2475–2477
Khalil KD, Al-Matar HM, Al-Dorri DM, Elnagdi MH (2009) Studies with enaminones and enaminonitriles: synthesis of 3-aroyl and 3-heteroaroyl-pyrazolo-[1,5-a]pyrimidines. Tetrahedron 65:9421–9427. doi:10.1016/j.tet.2009.08.084
Radl S, Blahovcova M, Tkadlecová M, Havlicek J (2010) Synthetic studies connected with the preparation of N-[3-(3-cyanopyrazolo[1,5-a]pyrimidin-5-yl)phenyl]-N-ethylacetamide, a zaleplon regioisomer. Heterocycles 80:1359–1379. doi:10.3987/COM-09-S(S)129
Goku A, Dolaz M, Digrak M, Serin S (2002) The biological activity of Dyer’s Madder. Proceedings of INCP 255
Acknowledgments
The authors wish to thank Rev. Fr. Dr. Stephen Mavely, Vice Chancellor, Assam Don Bosco University for providing infrastructure for the execution of this work. The authors also wish to express their gratitude to the IIT, Guwahati; Tezpur University, Tezpur; SAIF-NEHU, Shillong; and SAIF-CDRI, Lucknow. Our thanks are also due to the Department of Biotechnology (DBT), Government of India for a research Grant. SK thanks NER-BPMC-DBT, New Delhi for the award of a research fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kaping, S., Boiss, I., Singha, L.I. et al. A facile, regioselective synthesis of novel 3-(N-phenylcarboxamide)pyrazolo[1,5-a]pyrimidine analogs in the presence of KHSO\(_{4}\) in aqueous media assisted by ultrasound and their antibacterial activities. Mol Divers 20, 379–390 (2016). https://doi.org/10.1007/s11030-015-9639-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-015-9639-6