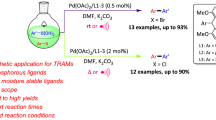
Abstract
An expedient catalytic method for the synthesis of diverse 7-(hetero) aryl-1H-pyrrolo[2,3-c]pyridine analogues via microwave-assisted Suzuki–Miyaura cross-coupling reaction with excellent yield was developed. The method is found to be compatible with various boronic acids and potassium organotrifluoroborates. The formation of highly stable monoligated catalytic species is found to be instrumental in driving the reactions to excellent conversions in Suzuki–Miyaura coupling. Herein, we report our findings on the use of a highly efficient precatalytic system (XPhos-PdG2), containing a bulky monodentate biaryl ligand which allows the rapid reductive elimination to form the true monoligated Pd(0) catalytic species, thereby facilitating the Suzuki coupling reaction of 7-chloro, 6-azaindole system containing unprotected free N–H group with excellent conversions employing low catalyst loadings. Also, we observed that the use of near stoichiometric potassium organotrifluoroborate reagents as alternative coupling partners for boronic acids, which are prone to protodeboronation, resulted in excellent conversions.
Graphical abstract








Similar content being viewed by others
References
Sajith AM, Abdul Khader KK, Joshi N, Nageswar Reddy M, Syed Ali Padusha M, Nagaswarupa HP, Nibin Joy M, Bodke YD, Karuvalam RP, Banerjee R, Muralidharan A, Rajendra P (2015) Design, synthesis and structure–activity relationship (SAR) studies of imidazo[4,5-b]pyridine derived purine isosteres and their potential as cytotoxic agents. Eur J Med Chem 89:21. https://doi.org/10.1016/j.ejmech.2014.10.037
Reddy EK, Remya C, Sajith AM, Dileep KV, Sadasivan C, Anwar S (2016) Functionalised dihydroazo pyrimidine derivatives from Morita–Baylis–Hillman acetates: synthesis and studies against acetylcholinesterase as its inhibitors. RSC Adv 6:77431. https://doi.org/10.1039/c6ra12507g
Reddy EK, Remya C, Mantosh K, Sajith AM, Omkumar RV, Sadasivan C, Anwar S (2017) Novel tacrine derivatives exhibiting improved acetylcholinesterase inhibition: design, synthesis and biological evaluation. Eur J Med Chem 139:367–377. https://doi.org/10.1016/j.ejmech.2017.08.013
Mérour Jean-Yves, Buron Frédéric, Plé Karen, Bonnet Pascal, Routier Sylvain (2014) The azaindole framework in the design of kinase inhibitors. Molecules 19:19935–19979. https://doi.org/10.3390/molecules191219935
Gummadi VR, Rajagopalan S, Looi C-Y, Paydar M, Renukappa GA, Ainan BR, Krishnamurthy NR, Panigrahi SK, Mahasweta K, Raghuramachandran S (2013) Discovery of 7-azaindole based anaplastic lymphoma kinase (ALK) inhibitors: wild type and mutant (L1196M) active compounds with unique binding mode. Bioorg Med Chem Lett 23:4911–4918. https://doi.org/10.1016/j.bmcl.2013.06.071
Pollard JR, Mortimore M (2009) Discovery and development of aurora kinase inhibitors as anticancer agents. J Med Chem 52:2629–2651. https://doi.org/10.1021/jm8012129
Bouloc N, Large JM, Kosmopoulou M, Sun C, Faisal A, Matteucci M, Reynisson J, Brown N, Atrash B, Blagg J (2010) Structure-based design of imidazo[1,2-a]pyrazine derivatives as selective inhibitors of Aurora-A kinase in cells. Bioorg Med Chem Lett 20:5988–5993. https://doi.org/10.1016/j.bmcl.2010.08.091
Harrington PE, Bourbeau MP, Fotsch C, Frohn M, Pickrell AJ, Reichelt A, Sham K, Siegmund AC, Bailis JM, Bush T (2013) The optimization of aminooxadiazoles as orally active inhibitors of Cdc7. Bioorg Med Chem Lett 23:6396–6400. https://doi.org/10.1016/j.bmcl.2013.09.055
Williams DK, Chen X-T, Tarby C, Kaltenbach R, Cai Z-W, Tokarski JS, An Y, Sack JS, Wautlet B, Gullo-Brown J (2010) Design, synthesis and structure–activity relationships of novel biarylamine-based Met kinase inhibitors. Bioorg Med Chem Lett 20:2998–3002. https://doi.org/10.1016/j.bmcl.2010.01.042
Kim KS, Zhang L, Schmidt R, Cai Z-W, Wei D, Williams DK, Lombardo LJ, Trainor GL, Xie D, Zhang Y (2008) Discovery of pyrrolopyridine–pyridone based inhibitors of Met kinase: synthesis, X-ray crystallographic analysis, and biological activities. J Med Chem 51:5330–5341. https://doi.org/10.1021/jm800476q
Pires MJD, Poeira DL, Purificacao SI, Marques MM (2016) Synthesis of substituted 4-, 5-, 6-, and 7-azaindoles from aminopyridines via a cascade C–N cross-coupling/heck reaction. Org Lett 18(13):3250–3253. https://doi.org/10.1021/acs.orglett.6b01500
Jean-Yves M, Benoît J (2001) Synthesis and reactivity of 7-azaindoles (1H-pyrrolo(2,3-b)pyridine). Curr Org Chem 5:471–506. https://doi.org/10.2174/1385272013375427
Florence P, Jean-Yves M, Benoıt J (2007) Synthesis and reactivity of 4-, 5- and 6-azaindoles. Tetrahedron 63:8689–8707. https://doi.org/10.1016/j.tet.2007.05.078
Henderson JL, McDermott SM, Buchwald SL (2010) Palladium-catalyzed amination of unprotected halo-7-azaindoles. Org Lett 12(20):4438–4441. https://doi.org/10.1021/ol101928m
Gaozhi C, Zhiguo L, Yali Z, Xiaoou S, Lili J, Yunjie Z, Wenfei H, Zhiguo F, Shulin Y, Guang L (2013) Synthesis and anti-inflammatory evaluation of novel benzimidazole and imidazopyridine derivatives. ACS Med Chem Lett 4(1):69–74. https://doi.org/10.1021/ml300282t
Takeuchi K, Bastian JA, Gifford-Moore DS, Harper RW, Miller SC, Mullaney JT, Sall DJ, Smith GF, Zhang M, Fisher MJ (2000) 1,2-Disubstituted indole, azaindole and benzimidazole derivatives possessing amine moiety: a novel series of thrombin inhibitors. Bioorg Med Chem Lett 10(20):2347–2351. https://doi.org/10.1016/S0960-894X(00)00454-6
Wang T, Ledeboer MW, Duffy JP, Salituro FG, Pierce AC, Zuccola HJ, Block E, Shlyakter D, Hogan JK, Bennani YL (2010) A novel chemotype of kinase inhibitors: discovery of 3,4-ring fused 7-azaindoles and deazapurines as potent JAK2 inhibitors. Bioorg Med Chem Lett 20(1):153–156. https://doi.org/10.1016/j.bmcl.2009.11.021
Alexander Düfert M, Kelvin Billingsley L, Stephen Buchwald L (2013) Suzuki–Miyaura cross-coupling of unprotected, nitrogen-rich heterocycles: substrate scope and mechanistic investigation. J Am Chem Soc 135(34):12877–12885. https://doi.org/10.1021/ja4064469
Sajith AM, Muralidharan A (2012) Exploration of copper and amine-free Sonogashira cross coupling reactions of 2-halo-3-alkyl imidazo[4,5-b]pyridines using tetrabutyl ammonium acetate as an activator under microwave enhanced conditions. Tetrahedron Lett 53:5206–5210. https://doi.org/10.1016/j.tetlet.2012.07.028
Sajith AM, Muralidharan A (2012) Microwave enhanced Suzuki coupling: a diversity-oriented approach to the synthesis of highly functionalised 3-substituted-2-aryl/heteroaryl imidazo[4,5-b]pyridines. Tetrahedron Lett 53:1036–1041. https://doi.org/10.1016/j.tetlet.2011.12.051
Sajith AM, Muralidharan A, Ranjith PK, Haridas K (2013) Cross-coupling reaction of 2-halo1-methyl-1H-imidazo[4,5-b]pyridine offers a new synthetic route to mutagenic heterocyclic amine-PHIP and DMIP. J Korean Chem Soc 57:3. https://doi.org/10.5012/jkcs.2013.57.3.361
Ranjith PK, Haridas K, Sajith AM, Muralidharan A (2013) A facile access to substituted indoles utilizing palladium catalyzed annulation under microwave enhanced conditions. Tetrahedron Lett 54(5126–512):9. https://doi.org/10.1016/j.tetlet.2013.07.073
Nibin Joy M, Yadav Bodke D, Abdul Khader KK, Syed Ali Padusha M, Sajith AM (2014) A rapid and modified approach for C-7 amination and amidation of 4-methyl-7-nonafluorobutylsulfonyloxy coumarins under microwave irradiation. RSC Adv 4:19766–19777. https://doi.org/10.1039/c4ra01720j
Nibin Joy M, Yadav Bodke D, Abdul Khader KK, Sajith AM, Venkatesh T, Ajeesh Kumar AK (2016) Simultaneous exploration of TBAF·3H2O as a base as well as a solvating agent for the palladium catalyzed Suzuki cross-coupling of 4-methyl-7-nonafluorobutylsulfonyloxy coumarins under microwave irradiation. J Fluor Chem 182:109–120. https://doi.org/10.1016/j.jfluchem.2016.01.002
Nagaraja RG, Reddy EK, Sajith AM, Shivaraj Y, Chandrashekar KB (2017) NMI/MsCl-mediated amide bond formation of aminopyrazines and aryl/heteroaryl carboxylic acids: synthesis of biologically relevant pyrazine carboxamides. ChemistrySelect 2:7706. https://doi.org/10.1002/slct.201700801
Abdul Khader KK, Sajith AM, Padusha MSA, Nagaswarupa HP, Muralidharan A (2014) Cycloalkenyl nonaflates as electrophilic cross-coupling substrates for palladium catalyzed C–N bond forming reactions with enolizable heterocycles under microwave enhanced conditions. New J Chem 38:1294–1305. https://doi.org/10.1039/c3nj01355c
Nibin Joy M, Yadav Bodke D, Abdul Khader KK, Syed Ali Padusha M, Sajith AM (2014) A rapid approach for the copper, amine, and ligand-free Sonogashira coupling of 4-methyl-7-nonafluorobutylsulfonyloxy coumarins under microwave irradiation. Tetrahedron Lett 55:2355–2361. https://doi.org/10.1016/j.tetlet.2014.02.094
Jin X, Guofeng Z, Lianghua Z, Yunyun L (2018) Direct synthesis of methylene-bridged bis-biaryl carboxylates via cascade Suzuki coupling and CH2Cl2-based bis-esterification. ChemistrySelect 3:8291–8293. https://doi.org/10.1002/slct.201801480
Xuwen C, Changfeng H, Jie-Ping W, Yunyun L (2016) Dichloromethane as methylene donor for the one-pot synthesis of bisaryloxy methanes via Williamson etherification and Suzuki coupling. Tetrahedron Lett 57:5116–5119. https://doi.org/10.1016/j.tetlet.2016.10.023
Manolikakes G, Hernandez CM, Schade MA, Metzger A, Knochel P (2008) Palladium- and nickel-catalyzed cross-couplings of unsaturated halides bearing relatively acidic protons with organozinc reagents. J Org Chem 73:8422–8436. https://doi.org/10.1021/jo8015852
Manolikakes G, Schade MA, Hernandez CM, Mayr H, Knochel P (2008) Negishi cross-couplings of unsaturated halides bearing relatively acidic hydrogen atoms with organozinc reagents. Org Lett 10:2765–2768. https://doi.org/10.1021/ol8009013
Manolikakes G, Dong Z, Mayr H, Li J, Knochel P (2009) Negishi cross-couplings compatible with unprotected amide functions. Chem Eur J 15:1324–1328. https://doi.org/10.1002/chem.200802349
Yang Y, Oldenhuis NJ, Buchwald SL (2013) Mild and general conditions for Negishi cross-coupling enabled by the use of palladacycle precatalysts. Angew Chem Int Ed 52:615–619. https://doi.org/10.1002/anie.201207750
Hooper A, Zambona A, Springer CJ (2016) A novel protocol for the one-pot borylation/Suzuki reaction provides easy access to hinge-binding groups for kinase inhibitors. Org Biomol Chem 14(3):963–969. https://doi.org/10.1039/C5OB01915J
Hwangseo P, Soyoung L, Suhyun L, Sungwoo H (2014) Structure-based de novo design and identification of D816V mutant-selective c-KIT inhibitors. Org Biomol Chem 12(26):4644–4655. https://doi.org/10.1039/C4OB00053F
Sera M, Mizufune H, Ueda T, Mineno M, Zanka A (2017) Integrated Pd-catalyzed cross-coupling strategies for furnishing α-carbolines. Tetrahedron 73:5946–5958. https://doi.org/10.1016/j.tet.2017.08.042
Buck JR, Saleh S, Uddin MI, Manning HC (2012) Rapid, microwave-assisted organic synthesis of selective (V600E)BRAF inhibitors for preclinical cancer research. Tetrahedron Lett 53:4161–4165. https://doi.org/10.1016/j.tetlet.2012.05.137
Ya-Ling Z, Zhen-Yu W, Yin-Mao F, You-Gui L, Kantchev EAB (2017) Solvent and base in one: tetra-n-butylammonium acetate as a multi-purpose ionic liquid medium for Ru-catalyzed directed mono- and di-o-C–H arylation reactions. Eur J Org Chem 42:6274–6282. https://doi.org/10.1002/ejoc.201701022
Savitha B, Reddy EK, Parthasarathi D, Rajeesh P, Sajith AM, Ananda Kumar CS, Haridas KR, Syed Ali Padusha M (2018) A highly efficient catalyst for the Suzuki–Miyaura cross-coupling reaction of 5-(5-chloropyridin-3-yl)-3-methyl-1,3,4-oxadiazol-2(3H)-one. J Heterocycl Chem 55(10):2277–2283. https://doi.org/10.1002/jhet.3280
Bulfield D, Huber SM (2017) Synthesis of polyflourinated biphenyls; pushing the boundaries of Suzuki–Miyaura cross coupling with electron-poor substrates. J Org Chem 82(24):13188–13203. https://doi.org/10.1021/acs.joc.7b02267
Molander GA, Trice SLJ, Kennedy SM (2012) Scope of the two-step, one-pot palladium-catalyzed borylation/Suzuki cross-coupling reaction utilizing bis-boronic acid. J Org Chem 77:8678. https://doi.org/10.1021/jo301642v
Bruneau A, Roche M, Alami M, Messaoudi S (2015) 2-aminobiphenyl palladacycles: the “most powerful” precatalysts in C–C and C–heteroatom cross-couplings. ACS Catal 5:1386–1396. https://doi.org/10.1021/cs502011x
Avinesh P, Jaison TJ, Sajith AM, Nagaswarupa HP, Muralidharan A (2016) Facile synthesis of fully decorated imidazo[4,5-b] and imidazo[4,5-c] pyridines in aqueous DMF via C–H activation under microwave irradiation. ChemistrySelect 1:2265–2270. https://doi.org/10.1002/slct.201600374
Xuwen C, Yunyun L (2018) Microwave-assisted decarbonylative coupling reaction of o-halobenzamides for phenanthridinone synthesis. ChemistrySelect 3:7763–7765. https://doi.org/10.1002/slct.201801441
Fontana M, Caseri W, Smith P (2006) Electro-spun, semiconducting, oriented fibres of supramolecular quasi-linear platinum compounds. Platin Met Rev 50(2):110. https://doi.org/10.1595/147106706x128412
Savitha B, Sajith AM, Reddy EK, Ananda Kumar CS, Ali Padusha MS (2016) Suzuki–Miyaura cross-coupling reaction in water: facile synthesis of (hetero) aryl uracil bases using potassiumorganotrifluoroborates under microwave irradiation. ChemistrySelect 1:4721–4725. https://doi.org/10.1002/slct.201600943
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Savitha, B., Reddy, E.K., Parthasarathi, D. et al. A highly efficient precatalytic system (XPhos-PdG2) for the Suzuki–Miyaura cross-coupling of 7-chloro-1H-pyrrolo[2,3-c]pyridine employing low catalyst loading. Mol Divers 23, 697–707 (2019). https://doi.org/10.1007/s11030-018-9904-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-018-9904-6