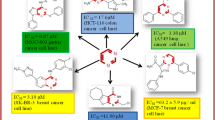
Abstract
Mono-/dispirocyclotriphosphazenes with pendant arm(s) are robust, but they are less investigated inorganic ring systems. In this study, a series of mono (3 and 4)- and dispirocyclotriphosphazenes with 4-chloro-benzyl pendant arm(s) (13–16) was obtained from the Cl exchange reactions of hexachlorocyclotriphosphazene with sodium (N-benzyl)aminopropanoxides (1 and 2). When compound (3) reacted with excess pyrrolidine, morpholine, tetra-1,4-dioxa-8-azaspiro[4,5]decane (DASD) and piperidine, the fully substituted monospirocyclotriphosphazenes (7, 9, 10 and 12) occurred. But, the reactions of 4 with excess piperidine and morpholine produced the gem-piperidino (5)- and morpholino (6)-substituted monospirocyclotriphosphazenes, whereas the reactions of 4 with excess pyrrolidine and DASD gave the fully substituted monospirocyclotriphosphazenes (8) and (11). However, it should be indicated that these derivatives were obtained to be used for the investigation of their spectral, stereogenic and biological properties. The structures of 5, 7 and 14 were determined crystallographically. X-ray data of 5 and 14 displayed that both of compounds were chiral in solid state, and their absolute configurations were assigned as R and RR. Additionally, the antimicrobial activities of phosphazenes were investigated. Minimum inhibitory concentrations, minimal bacterial concentrations and minimum fungicidal concentrations of phosphazenes were determined. The interactions of phosphazenes with plasmid DNA were evaluated by agarose gel electrophoresis. The cytotoxic activities of compounds were studied against L929 fibroblast and DLD-1 colon cancer cells. In addition, density functional theory calculations of 5, 7 and 14 were reported, and their molecular docking studies with DNA, E. coli DNA gyrase and topoisomerase IV were presented.
Graphic abstract











Similar content being viewed by others
References
Caminade AM, Hameau A, Majoral JP (2016) The specific functionalization of cyclotriphosphazene for the synthesis of smart dendrimers. DaltonTrans 45:1810–1822
Rothemund S, Teasdale I (2016) Preparation of polyphosphazenes: a tutorial review. Chem Soc Rev 45:5200–5215
Chandrasekhar V, Narayanan RS (2017) Phosphazenes, organophosphorus chem. The Royal Society of Chemistry (RSC Publishing), Cambridge, pp 342–417
Berberoğlu İ, Asmafiliz N, Kılıç Z, Hökelek T, Koç LY, Türk M, Soltanzade H, Dal H (2016) Phosphorus nitrogen compounds: part 34. Syntheses, structural investigations, cytotoxic and biological activities of spiro-ansa-spiro and spiro-bino-spiro tetrameric phosphazene derivatives. Inorg Chim Acta 446:75–86
Akbaş H, Okumuş A, Kılıç Z, Hökelek T, Süzen Y, Koç LY, Açık L, Çelik ZB (2013) Phosphorus–nitrogen compounds part 27. Syntheses, structural characterizations, antimicrobial and cytotoxic activities, and DNA interactions of new phosphazenes bearing secondary amino and pendant (4-fluorobenzyl)spiro groups. Eur J Med Chem 70:294–307
Chandrasekhar V, Narayanan RS, Mamidala R, Venkatasubbaiah K (2018) Phosphazenes, Organophosphorus Chem. The Royal Society of Chemistry (RSC Publishing), Cambridge, pp 363–424
Hayes RF, Allen CW (2016) The mechanism of a phosphazene-phosphazane rearrangement. Dalton Trans 45:2060–2068
Kumar D, Singh N, Keshav K, Elias AJ (2011) Ring-closing metathesis reactions of terminal alkene-derived cyclic phosphazenes. Inorg Chem 50(1):250–260
Nikovskii IA, Chistyakov EM, Tupikov AS (2018) Phosphazene-containing ligands and complexes on their base. Russ J Gen Chem 88(3):474–494
Yıldırım T, Bilgin K, Yenilmez Çiftçi G, Tanrıverdi Eçik E, Şenkuytu E, Uludağ Y, Tomake L, Kılıç A (2012) Synthesis, cytotoxicity and apoptosis of cyclotriphosphazene compounds as anti-cancer agents. Eur J Med Chem 52:213–220
Asmafiliz N (2014) Syntheses of chiral phosphazenes with stereogenic centers: NMR behavior in the presence of a chiral solvating agent. Heteroatom Chem 25:83–94
Asmafiliz N, Kılıç Z, Hökelek T, Koç LY, Açık L, Süzen Y, Öner Y (2013) Phosphorus–nitrogen compounds: part 26. Syntheses, spectroscopic and structural investigations, biological and cytotoxic activities, and DNA interactions of mono and bisferrocenylspirocyclotriphosphazenes. Inorg Chim Acta 400:250–261
Elmas G, Okumuş A, Koç LY, Soltanzade H, Kılıç Z, Hökelek T, Dal H, Açık L, Üstündağ Z, Dündar D, Yavuz M (2014) Phosphorus–nitrogen compounds. Part 29. Syntheses, crystal structures, spectroscopic and stereogenic properties, electrochemical investigations, antituberculosis, antimicrobial and cytotoxic activities and DNA interactions of ansa-spiro-ansa cyclotetraphosphazenes. Eur J Med Chem 87:662–676
Beşli S, Allen CW, Mutlu Balcı C, Kara G, Yeşilot S, Uslu A (2017) Stereochemical aspects of cyclotriphosphazenes: prochiral and pseudo-asymmetric phosphorus atoms. Polyhedron 135:49–59
Tümer Y, Asmafiliz N, Arslan G, Kılıç Z, Hökelek T (2019) Phosphorus-nitrogen compounds: Part 45. Vanillinato-substituted cis- and trans-bisferrocenyldispirocyclotriphosphazenes: syntheses, spectroscopic and crystallographic characterizations. J Mol Struct 1181:235–243
Asmafiliz N, Civan M, Özben A, Kılıç Z, Ramazanoğlu N, Açık L, Hökelek T (2018) Phosphorus-nitrogen compounds. Part 39. Syntheses and Langmuir-Blodgett thin films and antimicrobial activities of N/N and N/O spirocyclotriphosphazenes with monoferrocenyl pendant arm. Appl Organomet Chem 32(4):e4223
Allcock HR, Napierala ME, Cameron CG, O’Connor SJM (1996) Synthesis and characterization of ionically conducting alkoxy ether/alkoxy mixed-substituent poly(organophosphazenes) and their use as solid solvents for ionic conduction. Macromolecules 29:1951–1956
Gutru R, Peera SG, Bhat SD, Sahu AK (2016) Synthesis of sulfonated poly(bis(phenoxy)phosphazene) based blend membranes and its effect as electrolyte in fuel cells. Solid State Ionics 296:127–136
Wang XB, Tan AYX, Cho CM, He YQ, CB, Ji R, Xie HQ Tsai JWH, Xu JW, (2017) Synthesis and properties of cyclotriphosphazene and perfluoropolyether-based lubricant with polar functional groups. Lubric Sci 29(1):31–42
Greish YE, Bender JD, Lakshmi S, Brown PW, Allcock HR, Laurencin CT (2005) Low temperature formation of hydroxyapatite-poly(alkyl oxybenzoate)phosphazene composites for biomedical applications. Biomaterials 26:1–9
Klein R, Welna DT, Weikel A, Allcock HR, Runt J (2007) Counterion effects on ion mobility and mobile ion concentration of doped polyphosphazene and polyphosphazene Ionomers. Macromolecules 40:3990–3995
Sassus JL, Graffeuil M, Castera P, Labarre JF (1985) Covalent binding of non-effective diaziridinocyclotriphosphazenes to natural polyamines as tumor finders makes potential anticancer agents. Inorg Chim Acta 108:23–27
Asmafiliz N, Berberoğlu İ, Özgür M, Kılıç Z, Kayalak H, Açık L, Türk M, Hökelek T (2019) Phosphorus-nitrogen compounds: Part 46. The reactions of N3P3Cl6 with bidentate and monodentate ligands: The syntheses, structural characterizations, antimicrobial and cytotoxic activities, and DNA interactions of (N/N)spirocyclotriphosphazenes with 4-chlorobenzyl pendant arm. Inorg Chim Acta 495:118949
Tanrıkulu Gİ, Yakut Özgür M, Okumuş A, Kılıç Z, Hökelek T, Aydın B, Açık L (2019) Phosphorus-nitrogen compounds part 47: the conventional and microwave-assisted syntheses of dispirocyclotriphosphazene derivatives with (4-fluoro/4-nitrobenzyl) pendant arms: structural and stereogenic properties and DNA interactions. Inorg Chim Acta 490:179–189
Güven Kuzey N, Özgür M, Cemaloğlu R, Asmafiliz N, Kılıç Z, Açık L, Aydın B, Hökelek T (2020) Mono- and dispirocyclotriphosphazenes containing 4-bromobenzyl pendant arm(s): Synthesis, spectroscopy, crystallography and biological activity studies. J Mol Struct 1220:128658
İşcan Ö, Cemaloğlu R, Asmafiliz N, Kılıç Z, Açık L, Özbeden P, Hökelek T (2020) Synthesis and spectroscopic properties of (N/O) mono- and dispirocyclotriphosphazene derivatives with benzyl pendant arms: study of biological activity. Turk J Chem 44:15–30
Tümer Y, Asmafiliz N, Zeyrek CT, Kılıç Z, Açık L, Çelik SP, Türk M, Çağdaş Tunalı B, Ünver H, Hökelek T (2018) Syntheses, spectroscopic and crystallographic characterizations of cis- and trans-dispirocyclic ferrocenylphosphazenes: molecular dockings, cytotoxic and antimicrobial activities. New J Chem 42(3):1740–1756
Pektaş S, Bilge Koçak S, Başterzi NS, Kılıç Z, Zeyrek CT, Çoban B, Yıldız U, Çelik Ö (2018) spiro-Cyclotriphosphazenes containing 4-hydroxyphenylethyl pendant arm: syntheses, structural characterization and DNA interaction study. Inorg Chim Acta 474:51–65
Doğan H, Bahar MR, Çalışkan E, Tekin S, Uslu H, Akman F, Koran K, Sandal S, Görgülü AO (2020) Synthesis and spectroscopic characterizations of hexakis[(1-(4’-oxyphenyl)-3-(substituted-phenyl)prop-2-en-1-one)]cyclotriphosphazenes: their in vitro cytotoxic activity, theoretical analysis and molecular docking studies. J Biomol Struct Dyn. https://doi.org/10.1080/07391102.2020.1846621
Bruker program 1D WIN-NMR (release 6.0) and 2D WIN-NMR (release 6.1)
Bruker (2016) APEX3, SADABS, SAINT and SHELXTL, Madison, WI
Sheldrick GM (1997) SHELXS-97, SHELXL-97. University of Göttingen, Göttingen
Sheldrick GM (2008) A short history of SHELX. Acta Cryst Sect A Found Crystallogr 64:112–122
Farrugia LJ (1997) ORTEP-3 for Windows. J Appl Crystallogr 30:565–566
Man SE (2010) New Reactions of Cyclic Oxygen, Nitrogen and Sulfur Acetal Derivatives. Dissertation, University College London
Tümer Y, Asmafiliz N, Kılıç Z, Aydın B, Açık L, Hökelek T (2018) Phosphorus-nitrogen compounds: Part 43. Syntheses, spectroscopic characterizations and antimicrobial activities of cis- and trans-N/O dispirocyclotriphosphazenes containing ferrocenyl pendant arms. J Mol Struct 1173:885–893
Elmas G, Okumuş A, Hökelek T, Kılıç Z (2019) Phosphorus-nitrogen compounds. Part 52. The reactions of octachlorocyclotetraphosphazene with sodium 3-(N-ferrocenylmethylamino)-1-propanoxide: investigations of spectroscopic, crystallographic and stereogenic properties. Inorg Chim Acta 497:119106
Carriedo GA, García Alonso FJ, González PA, Menéndez JR (1998) Infrared and Raman spectra of the phosphazene high polymer [NP(O2C12H8)]n. J Raman Spectrosc 29(4):327–330
Cremer D, Pople JA (1975) General definition of ring puckering coordinates. J Am Chem Soc 97:1354–1358
Allen FH, Kennard O, Watson DG, Brammer L, Orpen AG, Taylor R (1987) Tables of bond lengths determined by X-ray and neutron diffraction. Part 1 Bond lengths in organic compounds. J Chem Soc Perkin Trans 2(12):1–19
Pidcock E (2005) Achiral molecules in non-centrosymmetric space groups. Chem Commun 27:3457–3459
Bullen GJ (1971) An improved determination of the crystal structure of hexachlorocyclotriphosphazene (phosphonitrilic chloride). J Chem Soc A: Inorg Phys Theor 56:1450–1453
Chaplin AB, Harrison JA, Dyson PJ (2005) Revisiting the electronic structure of phosphazenes. Inorg Chem 44:8407–8417
Asmafiliz N, Kılıç Z, Hayvalı Z, Açık L, Hökelek T, Dal H, Öner Y (2012) Phosphorus–nitrogen compounds. Part 23: syntheses, structural investigations, biological activities, and DNA interactions of new N/O spirocyclotriphosphazenes. Spectrochim Acta A 86:214–223
Hirshfeld HL (1977) Bonded-atom fragments for describing molecular charge densities. Theor Chim Acta 44:129–138
Spackman MA, Jayatilaka D (2009) Hirshfeld surface analysis. Cryst Eng Comm 11:19–32
Turner MJ, McKinnon JJ, Wolff SK, Grimwood DJ, Spackman PR, Jayatilaka D, Spackman MA (2017) CrystalExplorer17. The University of Western Australia, Crawley
Venkatesan P, Thamotharan S, Ilangovan A, Liang H, Sundius T (2016) Crystal structure, Hirshfeld surfaces and DFT computation of NLO active (2E)-2-(ethoxycarbonyl)-3-[(1-methoxy-1-oxo-3-phenylpropan-2-yl)amino] prop-2-enoic acid. Spectrochim Acta Part A 153:625–636
McKinnon JJ, Jayatilaka D, Spackman MA (2007) Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem Commun 37:3814–3816
Hartwar VR, Sist M, Jorgensen MRV, Mamakhel AH, Wang X, Hoffmann CM, Sugimoto K, Overgaard J, Iversen BB (2015) Quantitative analysis of intermolecular interactions in orthorhombic rubrene. IUCrJ 2:563–574
Tümer Y, Asmafiliz N, Kılıç Z, Hökelek T, Koç LY, Açık L, Yola ML, Solak AO, Öner Y, Dündar D, Yavuz M (2013) Phosphorus–nitrogen compounds: Part 28. Syntheses, structural characterizations, antimicrobial and cytotoxic activities, and DNA interactions of new phosphazenes bearing vanillinato and pendant ferrocenyl groups. J Mol Struct 1049:112–124
Tümer Y, Koç LY, Asmafiliz N, Kılıç Z, Hökelek T, Soltanzade H, Açık L, Yola ML, Solak AO (2015) Phosphorus–nitrogen compounds: part 30. Syntheses and structural investigations, antimicrobial and cytotoxic activities and DNA interactions of vanillinato-substituted NN or NO spirocyclic monoferrocenyl cyclotriphosphazenes. J Biol Inorg Chem 20:165–178
Okumuş A, Akbaş H, Kılıç Z, Koç LY, Açık L, Aydın B, Türk M, Hökelek T, Dal H (2016) Phosphorus–nitrogen compounds part 33: in vitro cytotoxic and antimicrobial activities, DNA interactions, syntheses, and structural investigations of new mono(4-nitrobenzyl)spirocyclotriphosphazenes. Res Chem Intermed 42:4221–4251
Nascimento GGF, Locatelli J, Freitas PC, Silva GL (2000) Antibacterial activity of plant extracts and phytochemicals on antibiotic resistant bacteria. Braz J Microbiol 31(4):247–256
Fleming I (1976) Frontier orbitals and organic chemical reactions. Wiley, London
Pearson RG (1986) Absolute electronegativity and hardness correlated with molecular orbital theory. Proc Natl Acad Sci USA 83:8440–8441
Koopmans T (1934) Über die Zuordnung von Wellenfunktionen und Eigenwerten zu den Einzelnen Elektronen Eines atoms. Physica 1:104–113
Acknowledgements
The authors acknowledge the Scientific and Technical Research Council of Turkey Grant No.116Z400. Z. K. thanks the Turkish Academy of Sciences (TÜBA) for partial support of this work. T. H. is grateful to Hacettepe University Scientific Research Project Unit (Grant No. 013 D04 602 004). The numerical calculations reported in this paper were fully/partially performed at TUBITAK ULAKBIM, High Performance and Grid Computing Center (TRUBA resources).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
İşcan, Ö., Cemaloğlu, R., Asmafiliz, N. et al. Phosphorus–nitrogen compounds: part 53—synthesis, characterization, cytotoxic and antimicrobial activity, DNA interaction and molecular docking studies of new mono- and dispirocyclotriphosphazenes with pendant arm(s). Mol Divers 26, 1077–1100 (2022). https://doi.org/10.1007/s11030-021-10231-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-021-10231-5