Abstract
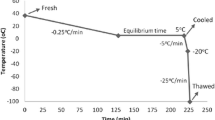
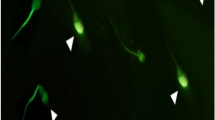
Use of cryopreserved semen has become an important tool in assisted reproduction but freezing and thawing cause sub-lethal damage to spermatozoa. This is detrimental to sperm because of the membrane damage including permeability and integrity. An excess generation of reactive oxygen species (ROS) creates oxidative stress due to reduced antioxidant status of the cryopreserved spermatozoa. In the present study fresh buffalo semen was collected and divided into two aliquots. One aliquot was used for fresh semen analysis and the other was cryopreserved in Tris-egg yolk-citrate extender. The semen samples were used to study different sperm quality parameters like motility, viability, membrane integrity and total antioxidant status. The DNA integrity in fresh and cryopreserved spermatozoa was also studied using comet assay. The sperm quality parameters like post-thaw sperm motility, viability, membrane integrity and total antioxidant status of cryopreserved spermatozoa were significantly lowered (P < 0.05) compared to fresh spermatozoa. The DNA fragmentation in cryopreserved spermatozoa was significantly higher (P < 0.01) as compared to fresh spermatozoa. The results show that the irreversible DNA damage occurs in spermatozoa during cryopreservation.







Similar content being viewed by others
Abbreviations
- ROS:
-
Reactive oxygen species
- DNA:
-
Deoxyribonucleic acid
References
Aitken RJ, Gordon E, Harkiss D, Twigg JP, Milne P, Jennings Z, Irvine DS (1998) Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol Reprod 59:1037–1046
Chatterjee S, Gagnon C (2001) Evidence for the production of oxygen free radicals during freezing/thawing of bull spermatozoa. Mol Reprod Dev 59(4):451–458
Salamon S, Maxwell WM (2000) Storage of ram semen. Anim Reprod Sci 62:77–111
Nandi S, Kumar GV, Chauhan MS (2006) In vitro production of bovine embryos: we need to stop or proceed-a review. Agric Rev 27:122–129
Totey SM, Singh G, Teneja M, Pawshe CH, Talwar GP (1992) In vitro maturation, fertilization and development of follicular oocytes from buffalo (Babalus bubalis). J Reprod Fertil 95:597–607
Vishwanath R, Shannon P (1995) The effect of optimal and suboptimal concentrations of sperm on the fertility of fresh and frozen bovine semen and a theoretical model to explain the fertility differences. Anim Reprod Sci 39:1–10
De Lamirande E, Gagnon C (1999) The dark and bright sides of reactive oxygen species on sperm function. In: Gagnon C (ed) The male gamete: from basic science to clinical applications. Cache River Press, Vienna, IL, pp 455–467
O’Flaherty C, Beorelgui B, Beconi MT (1999) Reactive oxygen species requirements for bovine sperm capacitation and acrosome reaction. Theriogenology 52:289–301
O’Flaherty C, Beorelgui B, Beconi MT (2005) Acrosome reaction in bovine spermatozoa: role of reactive oxygen species and lactate dehydrogenase C4. Biochim Biophys Acta 1726:96–101
Roy SC, Atreja SK (2007) Tyrosine phosphorylation in a 38-kDa capacitation associated buffalo (Bubalus bubalis) sperm proteins is induced by l-Arginine and regulated through a cAMP/PKA independent pathway. Int J Androl 31:12–24
Aitken RJ, Krausz C (2000) Oxidative stress, DNA damage and the Y chromosome. Reproduction 122:497–506
Kemal Duru N, Morshedi M, Oehninger S (2000) Effects of hydrogen peroxide on DNA and plasma membrane integrity of human spermatozoa. Fertil Steril 74:1200–1207
Kadirvel G, Kumar S, Kumaresan A (2009) Lipid peroxidation, mitochondrial membrane potential and DNA integrity of spermatozoa in relation to intracellular reactive oxygen species in liquid and frozen-thawed buffalo semen. Anim Reprod Sci 114(1–3):125–134
Nair JS, Brar AS, Ahuja CC, Sangha SPS, Chaudhary KC (2006) A comparative study on lipid peroxidation, activities of antioxidant enzymes and viability of cattle and buffalo bull spermatozoa during storage at refrigeration temperature. Anim Reprod Sci 96:21–29
Hughes CM, Lewis SEM, McKelvey-Martin VJ, Thompson W (1998) The effect of antioxidant supplementation during Percoll prepration on human sperm DNA integrity. Hum Reprod 13:115–154
Fraser L, Strzezek J (2004) The use of comet assay to assess DNA integrity of boar spermatozoa following liquid preservation at 5°C and 16°C. Folia Histochemica Et Cytobiologica 42(1):49–50
Tomar NS (1997) Artificial insemination and reproduction of cattle and buffaloes, 4th edn. Saroj Prakashan, Allahabad, India
Galantino-Homer HL, Visconti PE, Kopf GS (1997) Regulation of protein tyrosine phosphorylation during bovine sperm capacitation by a cyclic adenosine 3′, 5′-monophosphate-dependent pathway. Biol Reprod 56:707–719
Therien I, Manjunath P (2003) Effect of progesterone on bovine sperm capacitation and acrosome reaction. Biol Reprod 69:1408–1415
Revell SG, Mrode RA (1994) Anosmotic resistance test for bovine semen. Anim Reprod Sci 36:77–86
Benzie IEF, Strain JJ (1996) Ferric reducing/antioxidant power assay: direct measure of total anti oxidant activity of biological fluids and modified versions for simultaneous measurement of total antioxidant power and ascorbic acid concentration. In: Packer L (ed) Methods in enzymology, vol 299. Acadamic press, New York, pp 15–27
Singh NP, Charles HM, Richard EB (2003) Effects of age on DNA double-strand breaks and apoptosis in human sperm. Fertil Steril 80(6):1420–1430
Hammerstedt RH, Graham JK, Nolan JP (1990) Cryopreservation of mammalian sperm: what we ask them to survive. J Androl 11:73–88
Parks JE, Graham JK (1992) Effects of cryopreservation procedures on sperm membranes. Theriogenology 38:209–222
Isachenko V, Isachenko E, Katkov II, Montag M, Dessole S, Nawroth F, Vanderven H (2004) Cryoprotectant-free cryopreservation of human spermatozoa by vitrification and freezing in vapor: effects on motility, DNA integrity and fertilization ability. Biol Reprod 71:1167–1173
Lee Ying-Chu, Liao Chi-Jr, Pei-Tzn Li, Tzeng Woan-Fang, Chu Sin-Tak (2003) Mouse lipocalin as an enhancer of spermatozoa motility. Mol Biol Rep 30:165–172
Alvarez JG, Storey BT (1993) Evidence that membrane stress contributes more than lipid peroxidation to sublethal cryodamage in cryopreserved human sperm: glycerol and other polyols as sole cryoprotectant. J Androl 14:199–209
Chan SYW, Fox EJ, Chan MMC, Tsoi W, Wang C, Tang LCH, Tang GWK, Ho P (1985) The relationship between the human sperm hypoosmotic swelling, routine semen analysis, and the human sperm zona-free hamster ovum penetration assay. Fertil Steril 44:668–672
Lodhi LA, Zubair M, Qureshi ZI, Ahmad I, Jamil H (2008) Correlation between hypo-osmotic test and various conventional semen evaluation parameters in fresh and Nili-Ravi buffalo and Sahiwal cow bull semen. Pakistan Vet J 28(4):186–188
Spittaler PJ, Tyler JPP (1985) Further evaluation of a simple test for the integrity of spermatozoal membranes. Clin Reprod Fertil 3:187–196
Chan SYW, Wang C, Chan STH, Ho PC (1990) Differential evaluation of human sperm hypoosmotic swelling test and its relationship with the outcome of in vitro fertilization of human oocytes. Hum Reprod 5:84–88
Aitken RJ, Harkiss D, Buckingham D (1993) Relationship between iron-catalysed lipid peroxidation potential and human sperm function. J Reprod Fertil 98:257–265
Wang AW, Zhang H, Ikemoto I, Anderson DJ, Loughlin KR (1997) Reactive oxygen species generation by seminal cells during cryopreservation. Urology 49:921–925
Aitken RJ, Fisher JM, Fulton N, Gomez E, Knox W, Lewis B, Irvine S (1997) Reactive oxygen species generation by human spermatozoa is induced by exogenous NADPH and inhibited by the flavoprotein inhibitors diphenylene iodonium and quinacrine. Mol Reprod Dev 47:468–482
Tosic J (1947) Mechanism of hydrogen peroxide formation by spermatozoa and the role of amino-acids in sperm motility. Nature 159:544
Upreti GC, Jensen K, Munday R, Duganzich DM, Vishwanath R, Smith JF (1998) Studies on aromatic amino acid oxidase activity in ram spermatozoa: role of pyruvate as an antioxidant. Anim Reprod Sci 51:275–287
Shannon P, Curson B (1972) Toxic effect and action of dead sperm on diluted bovine semen. J Dairy Sci 55:614–620
Alvarez JG, Storey B (1989) Role of glutathione peroxidase in protecting mammalian spermatozoa from loss of motility caused by spontaneons lipid peroxidation. Gamete Res 23:77–90
Levis SE, Sterling S, Young IS, Thompson W (1997) Comparison of individual antioxidants and total antioxidant capacity of sperm and seminal plasma in fertile and infertile men. Fertil Steril 67:142–147
Alvarez JG, Touchstone JC, Blasco L, Storey BT (1987) Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa, superoxide dismutase as major enzyme protectant against oxygen toxicity. J Androl 8:338–348
Aitken RJ, Paterson M, Fisher H, Buckingham DW, Duin MV (1995) Redox regulation of tyrosine phosphorylation in human spermatozoa and its role in the control of human sperm function. J Cell Sci 108:2017–2025
Gadea J, Selles E, Marco EMA, Coy P, Matas C, Romar R, Ruiz S (2004) Decrease in glutathione content in boar sperm after cryopreservation, effect of the addition of reduced glutathione to the freezing and thawing extenders. Theriogenology 62:690–701
Bilodeau JF, Blanchette S, Gagnon C, Sirard MA (2001) Thiols prevent H2O2-mediated loss of sperm motility in cryopreserved bull semen. Theriogenology 56:275–286
Sailer BC, Jost IK, Evenson DP (1996) Bull sperm head morphometry related to abnormal chromatin structure and fertility. Cytometry 24:167–173
Aravindan GR, Bjordah IJ, Jost LK, Evenson DP (1997) Susceptibility of human sperm to in situ DNA denaturation is strongly correlated with DNA strand breaks identified by singly cell electrophoresis. Exp Cell Res 236:231–237
Hughes CM, Lewis SEM, McKelvey-Martin VJ, Thompson W (1996) A comparison of baseline and induced DNA damage in human sperm from fertile and infertile man, using a modified comet assay. Mol Hum Reprod 2:613–619
Hughes CM, Lewis SEM, McKelvey-Martin VJ, Thompson W (1998) The effects of antioxidant supplementation during Percoll preparation on human sperm DNA integrity. Mol Hum Reprod 13:1240–1247
Kodama H, Yamaguchi R, Fukuda J, Kasai H, Tanaka T (1997) Increased oxidative deoxyribonucleic acid damage in the spermatozoa of infertile male patients. Fertil Steril 68:519–524
Lopes S, Jurisicova A, Sun JG, Casper RF (1998) Reactive oxygen species: potential cause for DNA fragmentation in human spermatozoa. Hum Reprod 13:896–900
Lopes S, Sun JG, Jurisicova A, Meriano J, Casper RF (1998) Sperm deoxyribonucleic acid fragmentation is increased in poor-quality semen samples and correlates with failed fertilization in intracytoplasmic sperm injection. Fertil Steril 69:528–532
Fraser L, Strzezek J (2005) Effects of freezing–thawing on DNA integrity of boar spermatozoa assessed by the neutral comet assay. Reprod Domest Anim 40:530–536
Donnelly ET, McClure N, Lewis SE (2001) Cryopreservation of human semen and prepared sperm effects on motility parameters and DNA integrity. Fertil Steril 76(5):892–900
Menezo YJ, Hazout A, Panteix G, Robert F, Rollet J, Cohen-Bacrie P, Chapuis F, Clement P, Benkhalifa M (2007) Antioxidants to reduce sperm DNA fragmentation: an unexpected adverse effect. Reprod Biomed Online 14(4):418–421
Acknowledgment
We sincerely thank the Director, National Dairy Research Institute, Karnal, India, for providing financial assistance and necessary facilities during the course of study. This study was partly supported by World Bank funded National Agricultural Innovative Project (C4/C30014).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kumar, R., Jagan Mohanarao, G., Arvind et al. Freeze–thaw induced genotoxicity in buffalo (Bubalus bubalis) spermatozoa in relation to total antioxidant status. Mol Biol Rep 38, 1499–1506 (2011). https://doi.org/10.1007/s11033-010-0257-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-010-0257-1