Abstract
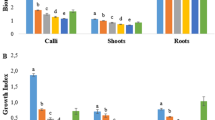
This study reports the effect of explant type and plant growth regulators (PGRs) on callus induction in Cnidium officinale. Compared to stem, root explant showed maximum percent callus formation of 75% on Murashige and Skoog (MS) medium containing 2.3 µM 2,4-dichlorophenoxyacetic acid (2,4-D) and 2.2 µM benzyladenine (BA). At 30th day of callus culture on the said medium, callus fresh weight was sevenfold higher than other tested PGRs treatments. It was noted that MS medium supplemented with 27.1 µM 2,4-D showed the highest 0.30 mg g−1 DW of total phenols, while total flavonoids content reached to a maximum of 0.05 mg g−1 DW on the MS medium supplemented with 4.5 µM 2,4-D and 2.2 µM BA. Conversely, maximum (83.9%) DPPH free radical scavenging activity was observed in calli grown on the MS medium supplemented with 2.3 µM 2,4-D and 2.2 µM BA. The high-performance liquid chromatography (HPLC) analysis revealed higher phthalide content in callus than intact roots of in vitro plants. While 3-butylidenephthalide content in callus was comparable to the intact shoots and roots of in vitro grown C. officinale. The concentrations of 2,4-D played a significant role in the production of phthalide and 3-butylidenephthalide. Additional measures are recommended to further enhance their production in vitro.
Graphical abstract






Similar content being viewed by others
References
Ramalingam M, Yong KP (2010) Free radical scavenging activities of Cnidium officinale Makino and Ligusticum chuanxiong Hort. methanolic extracts. Pharmacogn Mag 6:323–330
Kwak DH, Kim JK, Kim JY, Jeong HY, Keum KS, Han SH, Rho YI, Woo WH, Jung KY, Choi BK (2002) Anti-angiogenic activities of Cnidium officinale Makino and Tabanus bovinus. J Ethnopharmacol 81:373–379
Bae KE, Choi YW, Kim ST, Kim YK (2011) Components of rhizome extract of Cnidium officinale Makino and their In vitro biological effects. Molecules 16:8833
Jeong JW, Choi YH, Park C (2015) Induction of apoptosis by ethanol extract of Cnidium officinale in Human Leukemia U937 cells through activation of AMPK. J Life Sci 25:1255–1264
Lee Y, Lee Y, Kim J, Kim Y, Kim J (2015) Cinidium officinale and its bioactive compound, butylidenephthalide, inhibit laser-induced choroidal neovascularization in a rat model. Molecules 20:19728
Chiu SC, Chen SP, Huang SY, Wang MJ, Lin SZ, Harn HJ, Pang CY (2012) Induction of apoptosis coupled to endoplasmic reticulum stress in human prostate cancer cells by n-butylidenephthalide. PLoS ONE 7:e33742
Fu RH, Hran HJ, Chu CL, Huang CM, Liu SP, Wang YC, Lin YH, Shyu WC, Lin SZ (2011) Lipopolysaccharide-stimulated activation of murine DC2. 4 cells is attenuated by n-butylidenephthalide through suppression of the NF-κB pathway. Biotechnol Lett 33:903–910
Teng CM, Chen WY, Ko WC, Ouyang C (1987) Antiplatelet effect of butylidenephthalide. Biochim Biophys Acta (BBA)-Gen Sub 924:375–382
Ko WC, Sheu JR, Tzeng SH, Chen CM (1998) The selective antianginal effect without changing blood pressure of butylidenephthalide in conscious rats. Planta Med 64:229–232
Ko WC, Liao CC, Shih CH, Lei CB, Chen CM (2002) Relaxant effects of butylidenephthalide in isolated dog blood vessels. Planta Med 68:1004–1009
Mimura Y, Kobayashi S, Naitoh T, Kimura I, Kimura M (1995) The structure-activity relationship between synthetic butylidenephthalide derivatives regarding the competence and progression of inhibition in primary cultures proliferation of mouse aorta smooth muscle cells. Biol Pharm Bull 18:1203–1206
Kobayashi S, Nagasawa S, Yamamoto Y, Donghyo K, Bamba T, Fukusaki E (2012) Metabolic profiling and identification of the genetic varieties and agricultural origin of Cnidium officinale and Ligusticum chuanxiong. J Biosci Bioeng 114:86–91
Murch SJ, Saxena PK (2006) St. John’s wort (Hypericum perforatum L.): challenges and strategies for production of chemically-consistent plants. Can J Plant Sci 86:765–771
Souza JM, Berkov S, Santos AS (2014) Improvement of friable callus production of Boerhaavia paniculata Rich and the investigation of its lipid profile by GC/MS. Anais da Academia Brasileira de Ciências 86:1015–1027
Jafari A, Kahrizi D, Mansouri M (2016) Effects of plant growth regulators and explant on callus induction in pennyroyal (Mentha pulegium L.). Biharean Biol 10:134–136
Minaei Chenar H, Kahrizi H, Molsaghi M (2016) Callus induction is affected by explant type and plant growth regulators in chickpea (Cicer arietinum L.). Biharean Biol 10:28–32
Soorni J, Kahrizi D (2015) Effect of genotype, explant type and 2,4-D on cell dedifferentiation and callus induction in cumin (Cuminum cyminum L.) medicinal plant. J Appl Biotechnol Rep 2(3):265–270
Minaei H, Kahrizi D, Zebarjadi A (2015) Effect of plant growth regulators and explant type upon cell dedifferentiation and callus induction in chickpea (Cicer arietinum L.). J Appl Biotechnol Rep 2:241–244
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Ali M, Abbasi BH, Haq I (2013) Production of commercially important secondary metabolites and antioxidant activity in cell suspension cultures of Artemisia absinthium L. Ind Crops Prod 49:400–406
Blainski A, Lopes GC, De Mello JCP (2013) Application and analysis of the Folin Ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules 18:6852–6865
Chang CC, Yang MH, Wen HM, Chern JC (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10:178–182
Khan T, Abbasi BH, Khan MA, Shinwari ZK (2016) Differential effects of thidiazuron on production of anticancer phenolic compounds in callus cultures of Fagonia indica. Appl Biochem Biotechnol 179:46–58
Jeong JB, Ju SY, Park JH, Lee JR, Yun KW, Kwon ST, Lim JH, Chung GY, Jeong HJ (2009) Antioxidant activity in essential oils of Cnidium officinale Makino and Ligusticum chuanxiong hort and their inhibitory effects on DNA damage and apoptosis induced by ultraviolet B in mammalian cell. Cancer Epidemiol 33:41–46
Soorni J, Kahrizi D (2015) Effect of genotype, explant type and 2, 4-D on Cell dedifferentiation and callus induction in cumin (Cuminum cyminum) medicinal plant. J Appl Biotechnol Rep 2:265–270
Pant B, Kohda H, Namera A (1996) Clonal propagation of Cnidium officinale by shoot tip culture. Planta Med 62:281–283
Lee CY, Kim YK, Kim YS, Suh SY, Lee SY, Park SU (2009) Somatic embryogenesis and plant regeneration in Cnidium officinale Makino. J Med Plants Res 3:096–100
Maharik N, Elgengaihi S, Taha H (2009) Anthocyanin production in callus cultures of Crataegus sinaica boiss. Int J Acad Res 1:30–34
El-Baz FK, Mohamed AA, Ali SI (2010) Callus formation, phenolics content and related antioxidant activities in tissue culture of a medicinal plant colocynth (Citrullus colocynthis). Nova Biotechnol 10:79–94
Jeong JB, Park JH, Lee HK, Ju SY, Hong SC, Lee JR, Chung GY, Lim JH, Jeong HJ (2009) Protective effect of the extracts from Cnidium officinale against oxidative damage induced by hydrogen peroxide via antioxidant effect. Food Chem Toxicol 47:525–529
Hseu YC, Chang WH, Chen CS, Liao JW, Huang CJ, Lu FJ, Chia YC, Hsu HK, Wu JJ, Yang HL (2008) Antioxidant activities of Toona sinensis leaves extracts using different antioxidant models. Food Chem Toxicol 46:105–114
Misra BB, Dey S (2012) Comparative phytochemical analysis and antibacterial efficacy of in vitro and in vivo extracts from East Indian sandalwood tree (Santalum album L.). Lett Appl Microbiol 55:476–486
Misra BB, Dey S (2012) Phytochemical analyses and evaluation of antioxidant efficacy of in vitro callus extract of East Indian sandalwood tree (Santalum album L.). J Pharmacogn Phytochem 1(3):7–16
Acknowledgements
Xiuxia Ren, Dong Il Kang and Luc The Thi were supported by a scholarship from the BK21 Plus Program, the Ministry of Education, Republic of Korea. The support of the Korea Institute of Planning and Evaluation for technology in Food, Agriculture, Forestry and Fisheries (Project No.116057-03) is highly acknowledged.
Author information
Authors and Affiliations
Contributions
We the authors do consent that this work was an outcome of our joint efforts
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Rights and permissions
About this article
Cite this article
Adil, M., Ren, X., Kang, D.I. et al. Effect of explant type and plant growth regulators on callus induction, growth and secondary metabolites production in Cnidium officinale Makino. Mol Biol Rep 45, 1919–1927 (2018). https://doi.org/10.1007/s11033-018-4340-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-018-4340-3