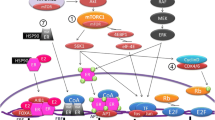
Abstract
Breast cancer is the most common cancer in women. It is a heterogeneous disease, encompassing different biological subtypes that differ in histological features, outcomes, clinical behaviour and different molecular subtypes. Therapy has progressed substantially over the past years with a reduction both for locoregional and systemic therapy. Endocrine therapies have considerably reduced cancer recurrence and mortality. Despite the major diagnostic and therapeutic innovations, resistance to therapy has become a main challenge, especially in metastatic breast cancer, and became a major factor limiting the use of endocrine therapeutic agents in ER positive breast cancers. Approximately 50% of patients with ER positive metastatic disease achieve a complete or partial response with endocrine therapy. However, in the remaining patients, the benefit is limited due to resistance, intrinsic or acquired, resulting in disease progression and poor outcome.
Tumour heterogeneity as well as acquired genetic changes and therapeutics pressure have been involved in the endocrine therapy resistance. Nowadays, targeted sequencing of genes involved in cancer has provided insights about genomic tumour evolution throughout treatment and resistance driver mutations. Several studies have described multiple alterations in receptor tyrosine kinases, signalling pathways such as Phosphoinositide-3-kinase–protein kinase B/Akt/mTOR (PI3K/Akt/mTOR) and Mitogen-activated protein kinase (MAPK), cell cycle machinery and their implications in endocrine treatment failure.
One of the current concern in cancer is personalized therapy. The focus has been the discovery of new potentially predictive biomarkers capable to identify reliably the most appropriate therapy regimen and which patients will experience disease relapse. The major concern is also to avoid overtreatment/undertreatment and development of resistance.
This review focuses on the most promising predictive biomarkers of resistance in estrogen receptor-positive breast cancer and the emerging role of circulating free-DNA as a powerful tool for longitudinal monitoring of tumour molecular profile throughout treatment.



Similar content being viewed by others
References
Globocan. Estimated number of new cases in 2018, Worldwide, all cancers, Females, all ages [Internet]. Vol. 849, Cancer Today. 2018. p. 2018. Available from: http://gco.iarc.fr/today/online-analysis-pie?v=2018&mode=cancer&mode_population=continents&population=900&populations=710&key=total&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=7&group_
Al-thoubaity FK (2020) Molecular classification of breast cancer: a retrospective cohort study. Ann Med Surg 49:44–48
Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P et al (2019) Breast cancer. Nat Rev Dis Prim 5:1–31
Viale G (2012) The current state of breast cancer classification. Ann Oncol 23:207–210
Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA et al (2000) Molecular portraits of human breast tumours. Nature 406:747–752
Szostakowska M, Trębińska-Stryjewska A, Grzybowska EA, Fabisiewicz A (2019) Resistance to endocrine therapy in breast cancer: molecular mechanisms and future goals. Breast Cancer Res Treat 173:489–497
Reinert T, Gonçalves R, Bines J (2018) Implications of ESR1 mutations in hormone receptor-positive breast cancer. Curr Treat Options Oncol 19:1–13
Reinert T, Barrios CH (2017) Overall survival and progression-free survival with endocrine therapy for hormone breast cancer : review. Ther Adv Med Oncol 9:693–709
Reinert T, Barrios CH (2015) Optimal management of hormone receptor positive metastatic breast cancer in 2016. Ther Adv Med Oncol 7:304–320
Hsu JL, Hung M-C (2016) The role of HER2, EGFR, and other receptor tyrosine kinases in breast cancer. Cancer Metastasis Rev 35:575–588
Vernieri C, Milano M, Brambilla M, Mennitto A, Maggi C, Cona MS et al (2019) Resistance mechanisms to anti-HER2 therapies in HER2-positive breast cancer: current knowledge, new research directions and therapeutic perspectives. Crit Rev Oncol Hematol 139:53–66
Clarke R, Tyson JJ, Dixon JM (2015) Endocrine resistance in breast cancer—an overview and update. Mol Cell Endocrinol 418:220–234
Haque MM, Desai KV (2019) Pathways to endocrine therapy resistance in breast cancer. Front Endocrinol (Lausanne) 10:1–7
Fang H, Huang D, Yang F, Guan X (2018) Potential biomarkers of CDK4/6 inhibitors in hormone receptor-positive advanced breast cancer. Breast Cancer Res Treat 168:287–297
Nicolini A, Ferrari P, Duffy MJ (2018) Prognostic and predictive biomarkers in breast cancer: past, present and future. Semin Cancer Biol 52:56–73
Bertucci F, Ng CKY, Patsouris A, Droin N, Piscuoglio S, Carbuccia N et al (2019) Genomic characterization of metastatic breast cancers. Nature 569(7757):560–564
Selli C, Dixon JM, Sims AH (2016) Accurate prediction of response to endocrine therapy in breast cancer patients: current and future biomarkers. Breast Cancer Res 18:1–10
Juric D, Castel P, Griffith M, Griffith OL, Won HH, Ellis H et al (2015) Convergent loss of PTEN leads to clinical resistance to a PI(3)Kα inhibitor. Nature 518:240–244
Lei JT, Gou X, Seker S, Ellis MJ (2019) ESR1 alterations and metastasis in estrogen receptor positive breast cancer. J Cancer Metastasis Treat 5:1–16
Zundelevich A, Dadiani M, Kahana-Edwin S, Itay A, Sella T, Gadot M et al (2020) ESR1 mutations are frequent in newly diagnosed metastatic and loco-regional recurrence of endocrine-treated breast cancer and carry worse prognosis. Breast Cancer Res 22:1–11
Rani A, Stebbing J, Giamas G, Murphy J (2019) Endocrine resistance in hormone receptor positive breast cancer-from mechanism to therapy. Front Endocrinol (Lausanne) 10:1–32
Hanker AB, Sudhan DR, Arteaga CL (2020) Overcoming endocrine resistance in breast cancer. Cancer Cell 37:496–513
Brett JO, Spring LM, Bardia A, Wander SA (2021) ESR1 mutation as an emerging clinical biomarker in metastatic hormone receptor-positive breast cancer. Breast Cancer Res 23:1–15
Fanning SW, Mayne CG, Dharmarajan V et al (2016) Estrogen receptor alpha somatic mutations Y537S and D538G confer breast cancer endocrine resistance by stabilizing the activating function-2 binding conformation. Elife. https://doi.org/10.7554/eLife.12792
Fiorillo M, Sanchez-Alvarez R, Sotgia F, Lisanti MP (2018) The ER-alpha mutation Y537S confers Tamoxifen-resistance via enhanced mitochondrial metabolism, glycolysis and Rho-GDI/PTEN signaling: Implicating TIGAR in somatic resistance to endocrine therapy. Aging (Albany NY) 10:4000–4023
Takeshita T, Yamamoto Y, Yamamoto-Ibusuki M, Tomiguchi M, Sueta A, Murakami K et al (2017) Analysis of ESR1 and PIK3CA mutations in plasma cell-free DNA from ER-positive breast cancer patients. Oncotarget 8:52142–52155
Clatot F, Perdrix A, Augusto L, Beaussire L, Delacour J, Calbrix C et al (2016) Kinetics, prognostic and predictive values of ESR1 circulating mutations in metastatic breast cancer patients progressing on aromatase inhibitor. Oncotarget 7:74448–74459
Zhang K, Hong R, Xu F, Xia W, Kaping L, Qin G et al (2018) Clinical value of circulating ESR1 mutations for patients with metastatic breast cancer: a meta-analysis. Cancer Manag Res 10:2573–2580
De Santo I, McCartney A, Migliaccio I, Di Leo A, Malorni L (2019) The emerging role of ESR1 mutations in luminal breast cancer as a prognostic and predictive biomarker of response to endocrine therapy. Cancers (Basel) 11:1–15
Allouchery V, Beaussire L, Perdrix A, Sefrioui D, Augusto L, Guillemet C et al (2018) Circulating ESR1 mutations at the end of aromatase inhibitor adjuvant treatment and after relapse in breast cancer patients. Breast Cancer Res 20:1–5
Schiavon G, Hrebien S, Garcia-murillas I, Cutts RJ, Tarazona N, Fenwick K et al (2015) Analysis of ESR1 mutation in circulating tumor DNA demonstrates evolution during therapy for metastatic breast cancer. Sci Transl Med 7:1–19
Gyanchandani R, Kota KJ, Jonnalagadda AR, Minteer T, Knapick BA, Oesterreich S et al (2017) Detection of ESR1 mutations in circulating cell-free DNA from patients with metastatic breast cancer treated with palbociclib and letrozole. Oncotarget 8:66901–66911
O’Leary B, Hrebien S, Morden JP, Beaney M, Fribbens C, Huang X et al (2018) Early circulating tumor DNA dynamics and clonal selection with palbociclib and fulvestrant for breast cancer. Nat Commun 9:1–10
Xi J, Ma CX (2020) Sequencing endocrine therapy for metastatic breast cancer: what do we do after disease progression on a CDK4/6 inhibitor? Curr Oncol Rep 22:1–12
Spoerke JM, Gendreau S, Walter K, Qiu J, Wilson TR, Savage H et al (2016) Heterogeneity and clinical significance of ESR1 mutations in ER-positive metastatic breast cancer patients receiving fulvestrant. Nat Commun 7:1–10
Sammons S, Shastry M, Dent S, Anders C, Hamilton E (2020) Practical treatment strategies and future directions after progression while receiving CDK4/6 inhibition and endocrine therapy in advanced HR+/HER2—breast cancer. Clin Breast Cancer 20:1–11
Brufsky AM, Dickler MN (2018) Estrogen receptor-positive breast cancer: exploiting signaling pathways implicated in endocrine resistance. Oncologist 5:528–539
Jhaveri K, Winer EP, Lim E, Fidalgo JA, Bellet M, Mayer IA, Boni V, Patel JM, Bardia A, Garcia JM, Kabos P, Gates M, Chen Y, Fredrickson J, Wang X, Friedman LS, Loi S (2020) A first-in-human phase I study to evaluate the oral selective estrogen receptor degrader (SERD), GDC-9545, in postmenopausal women with estrogen receptor-positive (ER+) HER2-negative (HER2-) metastatic breast cancer. Cancer Res. https://doi.org/10.1158/1538-7445.SABCS19-PD7-05
Paoletti C, Dolce EM, Schiavon G, Darga EP, Carr TH, Geradts J, Hoch M, Klinowska T, Lindemann J, Marshall G, Morgan S, Patel P, Rowlands V, Sathiyayogan N, Aung K, Baird R, Hayes DF (2018) Circulating biomarkers and resistance to endocrine therapy in metastatic breast cancers: correlative results from AZD9496 Oral SERD phase I trial. Clin Cancer Res 24:5860–72
Menyhárt O, Santarpia L, Győrffy B (2015) A comprehensive outline of trastuzumab resistance biomarkers in HER2 overexpressing breast cancer. Curr Cancer Drug Targets 15:665–683
Pearson A, Proszek P, Pascual J, Fribbens C, Shamsher MK, Kingston B et al (2020) Inactivating NF1 mutations are enriched in advanced breast cancer and contribute to endocrine therapy resistance. Clin Cancer Res 26:608–622
Razavi P, Chang MT, Xu G, Bandlamudi C, Ross DS, Vasan N et al (2018) The genomic landscape of endocrine-resistant advanced breast cancers. Cancer Cell 34:427–438
Sokol ES, Feng YX, Jin DX, Basudan A, Lee AV, Atkinson JM et al (2019) Loss of function of NF1 is a mechanism of acquired resistance to endocrine therapy in lobular breast cancer. Ann Oncol 30:115–123
O’Leary B, Finn RS, Turner NC (2016) Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol 13:417–430
Loibl S, Gianni L (2017) HER2-positive breast cancer. Lancet 389:2415–2429
Luque-Cabal M, García-Teijido P, Fernández-Pérez Y, Sánchez-Lorenzo L, Palacio-Vázquez I (2016) Mechanisms behind the resistance to trastuzumab in HER2-amplified breast cancer and strategies to overcome it. Clin Med Insights Oncol 10:21–30
Pernas S, Tolaney SM (2019) HER2-positive breast cancer: new therapeutic frontiers and overcoming resistance. Ther Adv Med Oncol 11:1–16
Vasan N, Toska E, Scaltriti M (2019) Overview of the relevance of PI3K pathway in HR-positive breast cancer. Ann Oncol 30:3–11
Keegan NM, Gleeson JP, Hennessy BT, Morris PG (2018) PI3K inhibition to overcome endocrine resistance in breast cancer. Expert Opin Investig Drugs 27:1–15
Collins D, Jacob W, Cejalvo JM, Ceppi M, James I, Weisser M et al (2017) Direct estrogen receptor (ER)/HER family crosstalk mediating sensitivity to lumretuzumab and pertuzumab in ER + breast cancer. PLoS ONE 12:1–16
Nahta R, Regan RMO, Crosstalk TÁ (2012) Therapeutic implications of estrogen receptor signaling in HER2-positive breast cancers. Breast Cancer Res Treat 135:39–48
Kaklamani VG, Richardson AL, Arteaga CL (2019) Exploring biomarkers of phosphoinositide 3-kinase pathway activation in the treatment of hormone receptor positive, human epidermal growth receptor 2 negative advanced breast cancer. Oncologist 24:305–312
Testa U, Castelli G, Pelosi E (2020) Breast cancer: a molecularly heterogenous disease needing subtype-specific treatments. Med Sci 8:1–103
Verret B, Cortes J, Bachelot T, Andre F, Arnedos M (2019) Efficacy of PI3K inhibitors in advanced breast cancer. Ann Oncol Off J Eur Soc Med Oncol 30:12–20
Schettini F, Buono G, Trivedi MV, De Placido S, Arpino G, Giuliano M (2017) PI3K/mTOR Inhibitors in the treatment of luminal breast cancer. Why When and to whom? Breast Care 12:290–4
Araki K, Miyoshi Y (2018) Mechanism of resistance to endocrine therapy in breast cancer: the important role of PI3K/Akt/mTOR in estrogen receptor-positive, HER2-negative breast cancer. Breast Cancer 25:392–401
van Geelen CT, Savas P, Teo ZL, Luen SJ, Weng C-F, Ko Y-A et al (2020) Clinical implications of prospective genomic profiling of metastatic breast cancer patients. Breast Cancer Res 22:1–13
Arthur LM, Turnbull AK, Renshaw L, Keys J, Thomas JS, Wilson TR et al (2014) Changes in PIK3CA mutation status are not associated with recurrence, metastatic disease or progression in endocrine-treated breast cancer. Breast Cancer Res Treat 147:211–219
Jacot W, Dalenc F, Lopez-Crapez E, Chaltiel L, Durigova A, Gros N et al (2019) PIK3CA mutations early persistence in cell-free tumor DNA as a negative prognostic factor in metastatic breast cancer patients treated with hormonal therapy. Breast Cancer Res Treat 177:659–667
Brandão M, Caparica R, Eiger D, de Azambuja E (2019) Biomarkers of response and resistance to PI3K inhibitors in estrogen receptor-positive breast cancer patients and combination therapies involving PI3K inhibitors. Ann Oncol 30:27–42
Arsenic R, Treue D, Lehmann A, Hummel M, Dietel M, Denkert C et al (2015) Comparison of targeted next-generation sequencing and Sanger sequencing for the detection of PIK3CA mutations in breast cancer. BMC Clin Pathol 15:1–9
Paul MR, Pan T, Pant DK, Shih NN, Chen Y, Harvey KL et al (2020) Genomic landscape of metastatic breast cancer identifies preferentially dysregulated pathways and targets. J Clin Invest 130:4252–4265
Mertins P, Mani DR, Ruggles KV, Gillette MA, Clauser KR, Wang P et al (2016) Proteogenomics connects somatic mutations to signalling in breast cancer. Nature 534:55–62
Kruger DT, Beelen KJ, Opdam M, Sanders J, van der Noort V, Boven E et al (2018) Hierarchical clustering of activated proteins in the PI3K and MAPK pathways in ER-positive, HER2-negative breast cancer with potential therapeutic consequences. Br J Cancer 119:832–839
André F, Hurvitz S, Fasolo A, Tseng L-M, Jerusalem G, Wilks S et al (2016) Molecular alterations and everolimus efficacy in human epidermal growth factor receptor 2–overexpressing metastatic breast cancers: combined exploratory biomarker analysis from BOLERO-1 and BOLERO-3. J Clin Oncol 34:2115–2124
Jankowitz RC, Oesterreich S, Lee AV, Davidson NE (2017) New strategies in metastatic hormone receptor-positive breast cancer: searching for biomarkers to tailor endocrine and other targeted therapies. Clin Cancer Res 23:1126–1131
Mosele F, Stefanovska B, Lusque A, Tran Dien A, Garberis I, Droin N et al (2020) Outcome and molecular landscape of patients with PIK3CA-mutated metastatic breast cancer. Ann Oncol 31:377–386
FDA approves first PI3K inhibitor for breast cancer | FDA [Internet]. [cited 2020 Sep 8]. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-pi3k-inhibitor-breast-cancer
Tzanikou E, Lianidou E (2020) The potential of ctDNA analysis in breast cancer. Crit Rev Clin Lab Sci 57:54–72
Lynce F, Shajahan-Haq AN, Swain SM (2018) CDK4/6 inhibitors in breast cancer therapy: current practice and future opportunities. Pharmacol Ther 191:65–73
Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im S, Masuda N et al (2016) Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phas. Lancet Oncol 17:425–439
McCartney A, Migliaccio I, Bonechi M, Biagioni C, Romagnoli D, De Luca F et al (2019) Mechanisms of resistance to CDK4/6 inhibitors: potential implications and biomarkers for clinical practice. Front Oncol 9:1–8
Costa C, Ye W, Ly A, Hosono Y, Murchi E, Walmsley CS et al (2020) Pten loss mediates clinical cross-resistance to CDK4/6 and PI3Kα inhibitors in breast cancer. Cancer Discov 10:72–85
Razavi P, Dickler MN, Shah PD, Toy W, Brown DN, Won HH et al (2020) Alterations in PTEN and ESR1 promote clinical resistance to alpelisib plus aromatase inhibitors. Nat Cancer 1:382–393
Skandalis SS, Afratis N, Smirlaki G, Nikitovic D, Theocharis AD, Tzanakakis GN et al (2014) Cross-talk between estradiol receptor and EGFR/IGF-IR signaling pathways in estrogen-responsive breast cancers : Focus on the role and impact of proteoglycans. Matrix Biol 35:182–193
Zhao Y, Zheng X, Zheng Y et al (2021) Extracellular matrix : emerging roles and potential therapeutic targets for breast cancer. Front Oncol 11:1–14
Saha T, Solomon J, Samson A, Gil-Henn H (2021) Invasion and metastasis as a central hallmark of breast cancer. J Clin Med 10:1–12
Afratis NA, Bouris P, Skandalis SS et al (2017) IGF-IR cooperates with ERα to inhibit breast cancer cell aggressiveness by regulating the expression and localisation of ECM molecules. Sci Rep 7:1–12
Bouris P, Skandalis SS, Piperigkou Z et al (2015) Estrogen receptor alpha mediates epithelial to mesenchymal transition, expression of specific matrix effectors and functional properties of breast cancer cells. Matrix Biol 43:42–60
Chen C, Gupta P, Parashar D et al (2020) ERBB3-induced furin promotes the progression and metastasis of ovarian cancer via the IGF1R/STAT3 signaling axis. Oncogene 36:2921–2933
Ianza A, Sirico M, Bernocchi O et al (2021) Role of the IGF-1 Axis in Overcoming Resistance in Breast Cancer. Front Cell Development Biol 9:1–9
Bendell JC, Jones SF, Hart L, Spigel DR, Lane CM, Earwood C et al (2015) A phase Ib study of linsitinib (OSI-906), a dual inhibitor of IGF-1R and IR tyrosine kinase, in combination with everolimus as treatment for patients with refractory metastatic colorectal cancer. Investig New Drugs 33:187–193
Barata P, Cooney M, Tyler A, Wright J, Dreicer R, Garcia JA (2018) A phase 2 study of OSI-906 (linsitinib, an insulin-like growth factor receptor-1 inhibitor) in patients with asymptomatic or mildly symptomatic (non-opioid requiring) metastatic castrate resistant prostate cancer (CRPC). Investig New Drugs 36:451–457
von Mehren M, George S, Heinrich MC, Schuetze SM, Yap JT, Yu JQ et al (2020) Linsitinib (OSI-906) for the treatment of adult and pediatric wild-type gastrointestinal stromal tumors, a SARC phase II study. Clin Cancer Res 26:1837–1845
Portman N, Alexandrou S, Carson E, Wang S, Lim E, Caldon CE (2019) Overcoming CDK4/6 inhibitor resistance in ER-positive breast cancer. Endocr Relat Cancer 26:R15-30
Niu Y, Xu J, Sun T (2019) Cyclin-dependent kinases 4/6 inhibitors in breast cancer: current status, resistance, and combination strategies. J Cancer 10:5504–5517
Shah M, Nunes MR, Stearns V (2018) CDK4/6 inhibitors: game changers in the management of hormone receptor-positive advanced breast cancer? Oncology (Williston Park) 32:216–222
Condorelli R, Spring L, O’Shaughnessy J, Lacroix L, Bailleux C, Scott V et al (2018) Polyclonal RB1 mutations and acquired resistance to CDK 4/6 inhibitors in patients with metastatic breast cancer. Ann Oncol 29:640–645
Johnson J, Thijssen B, Mcdermott U, Garnett M, Lodewyk FA (2016) Targeting the RB-E2F pathway in breast cancer. Oncogene 35:4829–4835
O’Leary B, Cutts RJ, Liu Y, Hrebien S, Huang X, Fenwick K et al (2018) The genetic landscape and clonal evolution of breast cancer resistance to palbociclib plus fulvestrant in the PALOMA-3 trial. Cancer Discov 8:1390–1403
Li Z, Razavi P, Li Q, Toy W, Liu B, Ping C et al (2018) Loss of the FAT1 tumor suppressor promotes resistance to CDK4/6 inhibitors via the Hippo pathway Zhiqiang. Cancer Cell 34:893–905
McCartney A, Bonechi M, De Luca F, Biagioni C, Curigliano G, Moretti E et al (2020) Plasma thymidine kinase activity as a biomarker in patients with luminal metastatic breast cancer treated with palbociclib within the TREnd trial. Clin Cancer Res 26:2131–2139
Bonechi M, Galardi F, Biagioni C, De Luca F, Bergqvist M, Neumüller M et al (2018) Plasma thymidine kinase-1 activity predicts outcome in patients with hormone receptor positive and HER2 negative metastatic breast cancer treated with endocrine therapy. Oncotarget 9:16389–16399
Ma CX, Gao F, Luo J, Northfelt DW, Goetz M, Forero A et al (2017) NeoPalAna: neoadjuvant palbociclib, a cyclin-dependent kinase 4/6 inhibitor, and anastrozole for clinical stage 2 or 3 estrogen receptor-positive breast cancer. Clin Cancer Res 23:4055–4065
Turner NC, Liu Y, Zhu Z, Loi S, Colleoni M, Loibl S et al (2019) Cyclin E1 expression and palbociclib efficacy in previously treated hormone receptor-positive metastatic breast cancer. J Clin Oncol 37:1169–1178
Guarducci C, Bonechi M, Benelli M, Biagioni C, Boccalini G, Romagnoli D et al (2018) Cyclin E1 and Rb modulation as common events at time of resistance to palbociclib in hormone receptor-positive breast cancer. npj Breast Cancer. 4:1–10
Wu Y, Zhang Y, Pi H, Sheng Y (2020) Current therapeutic progress of CDK4/6 inhibitors in breast cancer. Cancer Manag Res 12:3477–3487
Bagegni N, Thomas S, Liu N, Luo J, Hoog J, Northfelt DW et al (2017) Serum thymidine kinase 1 activity as a pharmacodynamic marker of cyclin-dependent kinase 4/6 inhibition in patients with early-stage breast cancer receiving neoadjuvant palbociclib. Breast Cancer Res 19:1–11
McCartney A, Biagioni C, Schiavon G, Bergqvist M, Mattsson K, Migliaccio I et al (2019) Prognostic role of serum thymidine kinase 1 activity in patients with hormone receptor–positive metastatic breast cancer: analysis of the randomised phase III evaluation of faslodex versus exemestane clinical trial (EFECT). Eur J Cancer 114:55–66
Saba R, Alsayed A, Zacny J, Dudek AZ (2016) The role of forkhead box protein M1 in breast cancer progression and resistance to therapy. Int J Breast Cancer 2016:1–9
Sanders DA, Ross-Innes C, Beraldi D et al (2013) Genome-wide mapping of FOXM1 binding reveals co-binding with estrogen receptor alpha in breast cancer cells. Genome Biol 14:1–16
Millour J, Constantinidou D, Stavropoulou AV, Wilson MSC et al (2010) FOXM1 is a transcriptional target of ERα and has a critical role in breast cancer endocrine sensitivity and resistance. Oncogene 29:2983–2995
Ahn H, Sim J, Abdul R et al (2015) Increased expression of forkhead Box M1 is associated with aggressive phenotype and poor prognosis in estrogen receptor- positive breast cancer. J Korean Med Sci 30:390–397
Madureira PA, Varshochi R, Constantinidou D et al (2006) The forkhead box M1 protein regulates the transcription of the estrogen receptor α in breast cancer cells. J Biol Chem 281:25167–25176
Bergamaschi A, Madak-Erdogan Z, Kim YJ et al (2014) The forkhead transcription factor FOXM1 promotes endocrine resistance and invasiveness in estrogen receptor-positive breast cancer by expansion of stem-like cancer cells. Breast Cancer Res 16:1–18
Lu XF, De Zeng, Liang W-Q et al (2015) FoxM1 is a promising candidate target in the treatment of breast cancer. Oncotarget 9:842–852
Bergamaschi A, Katzenellenbogen BS (2012) Tamoxifen down-regulation of miR-451 increases 14-3-3ζ and promotes breast cancer cell survival and endocrine resistance. Oncogene 31:39–47
Joshi S, Yang J, Wang Q, Li P, Wang H et al (2017) 14-3-3ζ loss impedes oncogene-induced mammary tumorigenesis and metastasis by attenuating oncogenic signaling. Am J Res 7:1654–1664
Bergamaschi A, Christensen BL, Katzenellenbogen BS (2011) Reversal of endocrine resistance in breast cancer: interrelationships among 14-3-3ζ, FOXM1, and a gene signature associated with mitosis. Breast Cancer Res 13:1–14
Parashar D, Nair B, Geethadevi A et al (2020) Peritoneal spread of ovarian cancer harbors therapeutic vulnerabilities regulated by FOXM1 and EGFR/ERBB2 signaling. Cancer Res 80:5554–5568
Li G, Zhao L, Li W, Fan K, Qian W (2014) Feedback activation of STAT3 mediates trastuzumab resistance via upregulation of MUC1 and MUC4 expression. Oncotarget 5:8317–8329
Sonnenblick A, Brohée S, Fumagalli D, Vincent D, Venet D, Ignatiadis M et al (2015) Constitutive phosphorylated STAT3-associated gene signature is predictive for trastuzumab resistance in primary HER2-positive breast cancer. BMC Med 13:1–10
West NR, Murphy LC, Watson PH (2012) Oncostatin M suppresses oestrogen receptor- a expression and is associated with poor outcome in human breast cancer. Endocr Relat Cancer 4:181–195
Canatan D, Yılmaz Ö, Sönmez Y, Çim A, Coşkun HŞ, Göksu SS et al (2021) Circulating microRNAs as potential non-invasive biomarkers for breast cancer detection. Acta Biomed 92:1–7
Parashar D, Geethadevi A, Aure MR, Mills GB, Pradeep S (2019) miRNA551b-3p activates an oncostatin signaling module for the progression of triple-negative breast cancer. Cell Rep 29:4389–4406
Chaluvally-raghavan P, Jin K, Pradeep S, George A, Sood AK, Mills GB et al (2016) Direct upregulation of STAT3 by MicroRNA-551b-3p deregulates growth and metastasis of ovarian. Cell Rep 15:1493–1504
Ji X, Lu Y, Tian H, Meng X et al (2019) Chemoresistance mechanisms of breast cancer and their countermeasures. Biomed Pharmacother 114:1–9
Wang T, Fahrmann JF, Lee H, Li Y-J et al (2018) JAK/STAT3-regulated fatty acid β-oxidation is critical for breast cancer stem cell self-renewal and chemoresistance. Cell Metab 27:136–150
Ma J, Qin L, Li X (2020) Role of STAT3 signaling pathway in breast cancer. Cell Commun Signal 18:1–13
Qin JJ, Yan L, Zhang J, Zhang WD (2019) STAT3 as a potential therapeutic target in triple negative breast cancer: a systematic review. J Exp Clin Cancer Res 38:16
Kohandel Z, Farkhondeh T, Aschner M, Mohammad A, Shahri P (2021) STAT3 pathway as a molecular target for resveratrol in breast cancer treatment. Cancer Cell Int 21:1–9
Pohlmann PR, Mayer IA, Mernaugh R (2009) Resistance to trastuzumab in breast cancer. Clin Cancer Res 15(24):7479–7491
Vu T, Claret FX (2012) Trastuzumab: updated mechanisms of action and resistance in breast cancer. Front Oncol 2:1–7
Chen AC, Migliaccio I, Rimawi M, Lopez-Tarruella S, Creighton CJ, Massarweh S, Huang C, Wang YC et al (2012) Upregulation of mucin4 in ER-positive/HER2-overexpressing breast cancer xenografts with acquired resistance to endocrine and HER2- targeted therapies. Breast Cancer Res Treat 134:583–593
Price-Schiavi SA, Jepson S, Li P, Arango M, Rudland PS, Yee L, Carraway KL (2002) Rat Muc4 (sialomucin complex) reduces binding of anti-ErbB2 antibodies to tumor cell surfaces, a potential mechanism for herceptin resistance. Int J Cancer 99:783–791
Luque-Cabal M, García-Teijido P, Fernández-Pérex Y et al (2016) Mechanisms behind the resistance to trastuzumab in HER2-amplified breast cancer and strategies to overcome it. Clinical Medicine Insights: Oncology 10:21–30
Gagliato DM, Jardim DLF et al (2016) Mechanisms of resistance and sensitivity to anti-HER2 therapies in HER2+ breast cancer. Oncotarget 7:64431–64446
Arnould L, Gelly M, Penault-Llorca F et al (2006) Trastuzumab-based treatment of HER2-positive breast cancer : an antibody-dependent cellular cytotoxicity mechanism? Br J Cancer 94:259–267
Miller TW, Rexer BN, Garrett JT, Arteaga CL (2011) Mutations in the phosphatidylinositol 3-kinase pathway: role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res 13:1–12
Sato F, Saji S, Toi M (2016) Genomic tumor evolution of breast cancer. Breast Cancer 23:4–11
Bertucci F, Ng CKY, Patsouris A, Droin N, Piscuoglio S, Carbuccia N et al (2019) Genomic characterization of metastatic breast cancers. Nature 569:560–564
Reinhardt F, Franken A, Fehm T, Neubauer H (2017) Navigation through inter- and intratumoral heterogeneity of endocrine resistance mechanisms in breast cancer: a potential role for Liquid Biopsies? Tumor Biol 39:1–15
Nicolini A, Ferrari P, Duffy MJ (2018) Prognostic and predictive biomarkers in breast cancer: past, present and future. Semin Cancer Biol 52:56–73
Zhang X, Ju S, Wang X, Cong H (2019) Advances in liquid biopsy using circulating tumor cells and circulating cell-free tumor DNA for detection and monitoring of breast cancer. Clin Exp Med 19:271–279
Hench IB, Hench J, Tolnay M (2018) Liquid biopsy in clinical management of breast, lung, and colorectal cancer. Front Med 5:1–24
Appierto V, Di Cosimo S, Reduzzi C, Pala V, Cappelletti V, Daidone MG (2017) How to study and overcome tumor heterogeneity with circulating biomarkers: the breast cancer case. Semin Cancer Biol 44:106–116
Fanning SW, Jeselsohn R, Dharmarajan V, Mayne CG, Karimi M, Buch-walter G, Houtman R, Toy W, Fowler CE, Han R et al (2018) The SERM/SERD bazedoxifene disrupts ESR1 helix 12 to overcome acquired hormone resistance in breast cancer cells. Elife. 2018(7):1–26
Kaklamani V, Bardia A, Wilks S, Weise A, Richards D, Harb W, Os-borne C, Wesolowski R, Karuturi M, Conkling P et al (2020) Abstract PD7-07: final analysis of phase 1 study of elacestrant (RAD1901), a novel selective estrogen receptor degrader (SERD), in estrogen receptor positive (ER+), human epidermal growth factor receptor 2 negative (HER2−) advanced breast cancer. Cancer Res. https://doi.org/10.1158/1538-7445.SABCS19-PD7-07
Laine M, Fanning SW, Greene M, Chang YF, Phung L, Tan TT, Hii-pakka R, Komm B, Greene G (2019) Lasofoxifene as a potential treat- ment for ER plus metastatic breast cancer. J Clin Oncol 37:1056
Andreano KJ, Wardell SE, Baker JG, Desautels TK, Baldi R, Chao CA, Heetderks KA, Bae Y, Xiong R, Tonetti DA et al (2020) G1T48, an oral selective estrogen receptor degrader, and the CDK4/6 inhibitor leroci- clib inhibit tumor growth in animal models of endocrine-resistant breast cancer. Breast Cancer Res Treat. https://doi.org/10.1007/s10549-020-05575-9
Funding
Not funds, grants, or other support was received.
Author information
Authors and Affiliations
Contributions
FM, FM, DM and FS drafted the manuscript, performed literature search and created the figures; HP conceived the idea; DM helped in designing the manuscript; DM and FM and FS critically revised the article and helped in the edition of the figures.
Corresponding author
Ethics declarations
Conflict of interest
The authors have stated that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Miranda, F., Prazeres, H., Mendes, F. et al. Resistance to endocrine therapy in HR + and/or HER2 + breast cancer: the most promising predictive biomarkers. Mol Biol Rep 49, 717–733 (2022). https://doi.org/10.1007/s11033-021-06863-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06863-3