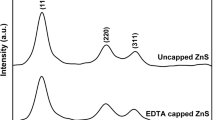
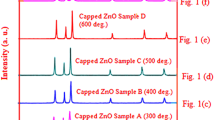
Abstract
The study of energy transfer mechanism from different capping agents to intrinsic luminescent vacancy centres of zinc sulphide (ZnS) has been reported in the present work. Nanoparticles of capped and uncapped ZnS are prepared by co-precipitation reaction. These nanoparticles are sterically stabilized using organic polymers—poly vinyl pyrrolidone, 2-mercaptoethanol and thioglycerol. Monodispersed nanoparticles were observed under TEM for both capped and uncapped ZnS nanopowders. However, for uncapped ZnS nanopowders, tendency for formation of nanorod like structure exists. Size of ZnS crystallites was calculated from X-ray diffraction pattern. The primary crystallite size estimated from X-ray diffraction pattern is 1.95–2.20 nm for capped nanostructures and 2.2 nm for uncapped nanostructures. FTIR spectra were conducted to confirm capping. Zeta potential measurements have been done to check the stability of dispersed nanoparticles. Band gap measurement was done by UV–visible spectrophotometer. Excitation and emission spectra are also performed in order to compare optical properties in various samples. Increase in emission intensity and band gap has been observed by adding different capping agents in comparison to uncapped ZnS nanoparticles. The results show that in capped ZnS nanoparticles the mechanism of energy transfer from capping layer to photoluminescent vacancy centres is more pronounced.









Similar content being viewed by others
References
Alivisatos AP, Harris TD, Carroll PJ, Stiegerwald ML, Brus LE (1989) Electron-vibration coupling in semiconductor clusters studied by resonance Raman spectroscopy. J Chem Phys 90:3463–3468
Bhargava RN (1996) Spectroscopy of isolated and assembled semiconductor nanocrystals special issue. J Lumin 70:85–94
British Pharmacopoeia (1993) Appendix IIB, A88-A89. HMSO, London
Denzler D, Olschewski M, Sattlera K (1998) Luminescence studies of localized gap states in colloidal ZnS nanocrystals. J Appl Phys 84(5):2841–2845
Dongjin K, Min K-D, Lee J, Park JH, Chun JH (2006) Influences of surface capping on particle size and optical characteristics of ZnS:Cu nanocrystals. Mater Sci Eng B 131:13–17
Ghosh G, Naskar MK, Patra A, Chatterjee M (2006) Synthesis and characterization of PVP-encapsulated ZnS nanoparticles. J Opt Mater 28:1047–1053
Gilbert B, Huang F, Lin Z, Goodell C, Zhang H, Banfield JF (2006) Surface chemistry controls crystallinity of ZnS nanoparticles. Nano Lett 6(4):605–610
Hullavarada NV, Hullavarad SS (2008) Optical properties of organic and inorganic capped CdS nanoparticles and the effects of x-ray irradiation on organic capped CdS nanoparticles. J Vac Sci Technol A 26:1050–1057
Kalele S, Gosavi SW, Urban J, Kulkarni SK (2006) Nanoshell particles: synthesis, properties and applications. Curr Sci 91(8):1038–1052
Kubo T, Isobe T, Senna M (2002) Enhancement of photoluminescence of ZnS:Mn nanocrystals modified by surfactants with phosphate or carboxyl groups via a reverse micelle method. J Lumin 99:39–45
Kumbhojkar N, Nikesh VV, Kshirsagar A (2000) Photophysical properties of ZnS nanoclusters. J Appl Phys 88(11):6260–6264
Manzoor K, Vadera SR, Kumar N, Kutty TRN (2003) Synthesis and photoluminescent properties of ZnS nanocrystals doped with copper and halogen. Mater Chem Phys 82:718–725
Manzoor K, Vadera SR, Kumar N, Kutty TRN (2004) Energy transfer from organic surface adsorbate-polyvinyl pyrrolidone molecules to luminescent centers in ZnS nanocrystals. Solid State Commun 129:469–473
Murase N, Hirata K, Yazawa T, Kushida T (1999) Fluorescence and EPR characteristics of Mn2+-doped ZnS nanocrystals prepared by aqueous colloidal method. J Phys Chem B 103:754–760
Murray CB, Kagan CR (2000) Synthesis and characterization of monodisperse nanocrystals and close-packed nanocrystal assemblies. Annu Rev Mater Sci 30:545–610
Suyver JF, Wuister SF, Kelly JJ, Meijerink A (2001) Synthesis and photoluminescence of nanocrystalline ZnS:Mn2+. Nano Lett 1:429–433
Warad HC, Ghosh SC, Hemtanon B, Thanachayanont C, Dutta J (2005) Luminescent nanoparticles of Mn doped ZnS passivated with sodium hexametaphosphate. Sci Technol Adv Mater 6:296–301
Yanagida S, Ishimaru Y, Miyake Y, Shiragami T, Pac C, Hashimoto K, Sakata T (1989) J Phys Chem 93:2576–2582
Zewei Q et al (2009) Multicolor tuning of manganese-doped ZnS colloidal nanocrystals. Langmuir 25:10259–10262
Acknowledgements
The authors are thankful to DRDO, New Delhi for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, M., Kumar, S. & Pandey, O.P. Study of energy transfer from capping agents to intrinsic vacancies/defects in passivated ZnS nanoparticles. J Nanopart Res 12, 2655–2666 (2010). https://doi.org/10.1007/s11051-009-9844-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-009-9844-2