Abstract
A phase II study was conducted to assess the efficacy of administering daily thalidomide concomitantly with radiation and continuing for up to 1 year following radiation in children with brain stem gliomas (BSG) or glioblastoma multiforme (GBM). Secondary objectives were to obtain preliminary evidence of biologic activity of thalidomide and to evaluate toxicities from chronic administration of thalidomide in this population.
Thirteen patients (2–14 years old) with newly diagnosed BSG (12 patients) or GBM (one patient) were enrolled between July 1999 and June 2000. All patients received focal radiotherapy to a total dose of 5,580 cGy. Thalidomide was administered once daily beginning on the first day of radiation and continued for 12 months or until the patient came off study. The starting dose was 12 mg/kg (rounded down to the nearest 50 mg) and was increased by 20% weekly, if tolerated, to 24 mg/kg or 1,000 mg (whichever was lower). Advanced imaging techniques and urine and serum analysis for anti-angiogenic markers were performed in some patients in an attempt to correlate changes with clinical effect of therapy.
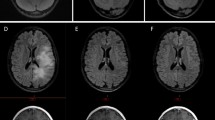
No patients completed the planned 12 months of thalidomide therapy and all have since died of disease progression. The median duration of therapy was 5 months (range 2–11 months). Nine patients came off study for progressive disease (PD), three patients due to toxicity and one patient withdrew consent. Several patients on this study required more extended courses of high dose steroids than would have been otherwise expected for this population due to significant peritumoral edema and necrosis. No consistent pattern emerged from the biologic correlative studies from 11 patients. However, advanced imaging with techniques such as MR spectroscopy, MR perfusion and 18-fluorodeoxyglucose positron emission tomography (FDG-PET) were helpful in distinguishing growing tumor from treatment effect and necrosis in some patients.
The median time to progression (TTP) was 5 months (range 2–11 months) and the median time to death (TTD) was 9 months (range 5–17 months). In this small patient sample adding thalidomide to radiation did not improve TTP or TTD from historical controls, however, toxicity appeared to be increased.

Similar content being viewed by others
Abbreviations
- DFCHCC:
-
Dana-Farber/Children’s Hospital Cancer Center
- CNMC:
-
Children’s National Medical Center
- GBM:
-
Glioblastoma multiforme
- BSG:
-
Brain stem glioma
- LDH:
-
Lactate dehydrogenase
- PD:
-
Progressive disease
- SD:
-
Stable disease
- PR:
-
Partial response
- CR:
-
Complete response
- bFGF:
-
Basic fibroblastic growth factor
- PDGF:
-
Platelet derived growth factor
- VEGF:
-
Vascular endothelial growth factor
- S.T.E.P.S.® :
-
System for Thalidomide Education and Prescribing Safety
- FDG PET:
-
18-Fluorodeoxyglucose positron emission tomography
- TTP:
-
Time to progression
- TTD:
-
Time to death
References
Jennings MT, Sposto R, Boyett JM et al (2002) Preradiation chemotherapy in primary high-risk brainstem tumors: phase II study CCG-9941 of the Children’s Cancer Group. J Clin Oncol 20:3431–3437
Freeman CR, Kepner J, Kun LE et al (2000) A detrimental effect of a combined chemotherapy-radiotherapy approach in children with diffuse intrinsic brain stem gliomas? Int J Radiat Oncol Biol Phys 47:561–564
Marcus KJ, Dutton SC, Barnes P et al (2003) A phase I trial of etanidazole and hyperfractionated radiotherapy in children with diffuse brainstem glioma. Int J Radiat Oncol Biol Phys 55:1182–1185
Benesch M, Lackner H, Moser A et al (2001) Outcome and long-term side effects after synchronous radiochemotherapy for childhood brain stem gliomas. Pediatr Neurosurg 35:173–180
Walker DA, Punt JA, Sokal M (1999) Clinical management of brain stem glioma. Arch Dis Child 80:558–564
Freeman CR, Perilongo G (1999) Chemotherapy for brain stem gliomas. Childs Nerv Syst 15:545–553
Fine HA, Figg WD, Jaeckle K et al (2000) Phase II trial of the antiangiogenic agent thalidomide in patients with recurrent high-grade gliomas. J Clin Oncol 18:708–715
Fanelli M, Sarmiento R, Gattuso D et al (2003) Thalidomide: a new anticancer drug? Expert Opin Investig Drugs 12:1211–1225
Fine HA, Wen PY, Maher EA et al (2003) Phase II trial of thalidomide and carmustine for patients with recurrent high-grade gliomas. J Clin Oncol 21:2299–2304
Crane E, List A (2005) Immunomodulatory drugs. Cancer Invest 23:625–634
Teo SK (2005) Properties of thalidomide and its analogues: implications for anticancer therapy. Aaps J 7:E14–E19
Mitsiades N, Mitsiades CS, Poulaki V et al (2002) Apoptotic signaling induced by immunomodulatory thalidomide analogs in human multiple myeloma cells: therapeutic implications. Blood 99:4525–4530
Fleming FJ, Vytopil M, Chaitow J et al (2005) Thalidomide neuropathy in childhood. Neuromuscul Disord 15:172–176
Short SC, Traish D, Dowe A et al (2001) Thalidomide as an anti-angiogenic agent in relapsed gliomas. J Neurooncol 51:41–45
Cha S (2006) Dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging in pediatric patients. Neuroimag Clin N Am 16:137–147, ix
Freeman CR, Farmer JP (1998) Pediatric brain stem gliomas: a review. Int J Radiat Oncol Biol Phys 40:265–271
Carrie C, Negrier S, Gomez F et al (2004) Diffuse medulla oblongata and pontine gliomas in childhood. A review of 37 cases. Bull Cancer 91:E167–E183
Farmer JP, Montes JL, Freeman CR et al (2001) Brainstem Gliomas. A 10-year institutional review. Pediatr Neurosurg 34:206–214
Kaplan AM, Albright AL, Zimmerman RA et al (1996) Brainstem gliomas in children. A Children’s Cancer Group review of 119 cases. Pediatr Neurosurg 24:185–192
Packer RJ, Boyett JM, Zimmerman RA et al (1993) Hyperfractionated radiation therapy (72 Gy) for children with brain stem gliomas. A Childrens Cancer Group Phase I/II Trial. Cancer 72:1414–1421
Reddy AT, Mapstone TB (2000) Brain stem tumors. In: Bernstein M, Berger MS (eds) Neuro-oncology: the essentials. Thieme Medical Publishers, Inc., New York, pp 352–362
Baumann F, Bjeljac M, Kollias SS et al (2004) Combined thalidomide and temozolomide treatment in patients with glioblastoma multiforme. J Neurooncol 67:191–200
Chang SM, Lamborn KR, Malec M et al (2004) Phase II study of temozolomide and thalidomide with radiation therapy for newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys 60:353–357
Acknowledgements
A preliminary report of data from this study was presented in part at the Tenth International Symposium for Pediatric Neuro-Oncology in June 2002. Support for this study was provided through the Stop & Shop Family Pediatric Brain Tumor Program. Costs of data management were supported by Celgene Pharmaceuticals Corporation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Turner, C.D., Chi, S., Marcus, K.J. et al. Phase II study of thalidomide and radiation in children with newly diagnosed brain stem gliomas and glioblastoma multiforme. J Neurooncol 82, 95–101 (2007). https://doi.org/10.1007/s11060-006-9251-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-006-9251-9