Abstract
Introduction
Alterations in the CDK4/6—RB signaling pathway are common causes of cell cycle dysregulation in many cancers, including glioblastoma. Palbociclib is an oral inhibitor of CDK4/6, which leads to phosphorylation of RB1 and cell-cycle arrest. We conducted a two-arm study evaluating efficacy and tissue pharmacokinetics/pharmacodynamics of palbociclib in patients with recurrent glioblastoma.
Methods
Eligibility criteria included confirmation of RB1 proficiency by IHC; ≤ 3 relapses; KPS ≥ 60; no limit on prior treatments. Arm 1 received palbociclib for 7 days prior to indicated resection followed by adjuvant palbociclib. Arm 2 received palbociclib without resection. Primary objective was PFS6; secondary included toxicity, OS, and ORR. Exploratory aims included biomarker assessment and pharmacokinetic/pharmacodynamic effects in surgical patients.
Results
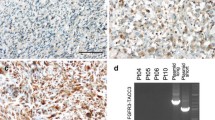
Total of 22 patients were enrolled; 6 on Arm 1 and 16 on Arm 2. Trial was stopped early secondary to lack of efficacy, with 95% of evaluable patients progressing within 6 months. Median PFS was 5.14 weeks (range 5 days–142 weeks) and median OS was 15.4 weeks (range 2–274 weeks). Two patients (10%) had related grade ≥ 3 AEs. In Arm 1, 5 patients had tissue concentrations of palbociclib felt to be sufficient for biological effect and paired samples available for RB1 IHC. There were no consistent changes in RB1 expression or cell proliferation in the paired tissue.
Conclusion
In this trial, despite adequate tissue PK, palbociclib monotherapy was not an effective treatment for recurrent glioblastoma. However, these were heavily pretreated patients and targeting the CDK4/6 pathway may still deserve further exploration.


Similar content being viewed by others
References
Stupp R et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996
Stupp R et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10(5):459–466
Friedman HS et al (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27(28):4733–4740
Kreisl TN et al (2009) Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27(5):740–745
Malumbres M, Barbacid M (2009) Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer 9(3):153–166
Cancer Genome Atlas Research Network (2008) Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455(7216):1061–1068
Michaud K et al (2010) Pharmacologic inhibition of cyclin-dependent kinases 4 and 6 arrests the growth of glioblastoma multiforme intracranial xenografts. Cancer Res 70(8):3228–3238
Finn RS et al (2016) Palbociclib and letrozole in advanced breast cancer. N Engl J Med 375(20):1925–1936
Wen PY et al (2010) Response assessment challenges in clinical trials of gliomas. Curr Oncol Rep 12(1):68–75
Goldhoff P et al (2012) Clinical stratification of glioblastoma based on alterations in retinoblastoma tumor suppressor protein (RB1) and association with the proneural subtype. J Neuropathol Exp Neurol 71(1):83–89
Clarke JL et al (2011) Is surgery at progression a prognostic marker for improved 6-month progression-free survival or overall survival for patients with recurrent glioblastoma? Neuro Oncol 13(10): 1118–1124
Lamborn KR et al (2008) Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol 10(2):162–170
PD-0332991 Investigator’s Brocure. 2010
Chinot OL et al (2014) Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 370(8):709–722
Gilbert MR et al (2014) A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370(8):699–708
Hashizume R et al (2016) Inhibition of DNA damage repair by the CDK4/6 inhibitor palbociclib delays irradiated intracranial atypical teratoid rhabdoid tumor and glioblastoma xenograft regrowth. Neuro Oncol 18:1519–1528
Sherr CJ, Beach D, Shapiro GI (2016) Targeting CDK4 and CDK6: from discovery to therapy. Cancer Discov 6(4):353–367
Huillard E et al (2012) Cooperative interactions of BRAFV600E kinase and CDKN2A locus deficiency in pediatric malignant astrocytoma as a basis for rational therapy. Proc Natl Acad Sci USA 109(22):8710–8715
Hashizume R et al (2016) Inhibition of DNA damage repair by the CDK4/6 inhibitor palbociclib delays irradiated intracranial atypical teratoid rhabdoid tumor and glioblastoma xenograft regrowth. Neuro Oncol 18(11):1519–1528
Goel S et al (2017) CDK4/6 inhibition triggers anti-tumour immunity. Nature 548(7668):471–475
Funding
Support for this study was provided by Pfizer (M.P.), National Cancer Institute P50CA097257 (M.P. and C.D.J), R01CA159467 (C.D.J.) and the Accelerated Brain Cancer Cure (ABC2) (M.P.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors report no conflicts of interest relevant to this study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Taylor, J.W., Parikh, M., Phillips, J.J. et al. Phase-2 trial of palbociclib in adult patients with recurrent RB1-positive glioblastoma. J Neurooncol 140, 477–483 (2018). https://doi.org/10.1007/s11060-018-2977-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-2977-3