Abstract
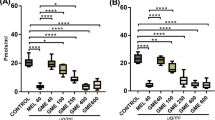
Parkinson’s disease (PD) is a neurodegenerative disorder and these days a lot of emphasis is given on the treatment of this disease using herbal medicines. The present study evaluates the neuroprotective effect of Withania somnifera (Ws) root extract on Parkinsonian mice. The mice were divided into three groups; the first group served as control, the second group was given maneb (MB) and paraquat (PQ) and the last group was administered MB–PQ along with Ws root extract for 3, 6 and 9 weeks. The behavioral studies showed a significant improvement in the motor movement patterns and gripping ability of Ws root extract exposed Parkinsonian mice. Tyrosine hydroxylase (TH) immunostaining was reduced in the substantia nigra of MB–PQ exposed mice, while Ws co-exposure restored TH immunostaining significantly. Additionally, our results also demonstrate generation of oxidative stress in the nigrostriatal region of MB–PQ exposed mice. There was a marked decline in the level of catalase and a simultaneous increase in the level of nitrite and lipid peroxidation in Parkinsonian mice. Thus, the Ws root extract have shown to counteract the pro-oxidants and their associated oxidative stress in the PD model studied here. Our results clearly indicate the usefulness of Ws root extract in providing protection against MB–PQ induced nigrostriatal dopaminergic neurodegeneration and marked improvement in the behavioral, anatomical and the biochemical deformities.







Similar content being viewed by others
References
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39:889–909
Mounsey RB, Teismann P (2010) Mitochondrial dysfunction in Parkinson’s disease pathogenesis and neuroprotection. Parkinsons Dis 2011:1–18
Fahn S (2000) The spectrum of levodopa-induced dyskinesias. Ann Neurol 4:2–11
Singh MP, Patel S, Dikshit M, Gupta YK (2006) Contribution of genomics and proteomics in understanding the role of modifying factors in Parkinson’s disease. Indian J Biochem Biophys 43:69–81
Singh C, Ahmad I, Kumar A (2007) Pesticides and metals induced Parkinson’s disease: involvement of free radicals and oxidative stress. Cell Mol Biol 53:19–28
Thiruchelvam M, Brockel BJ, Richfield EK, Baggs RB, Cory-Slechta DA (2000) Potentiated and preferential effects of combined paraquat and maneb on nigrostriatal dopamine systems: environmental risk factors for Parkinson’s disease? Brain Res 873:225–234
Cory-Slechta DA, Thiruchelvam M, Barlow BK, Richfield EK (2005) Developmental pesticide models of the Parkinson disease phenotype. Environ Health Perspect 113:1263–1270
Patel S, Singh V, Kumar A, Gupta YK, Singh MP (2006) Status of antioxidant defense system and expression of toxicant responsive genes in striatum of maneb-and paraquat-induced Parkinson’s disease phenotype in mouse: mechanism of neurodegeneration. Brain Res 1081:9–18
Uversky VN (2004) Neurotoxicant-induced animal models of Parkinson’s disease: understanding the role of rotenone, maneb and paraquat in neurodegeneration. Cell Tissue Res 318:225–241
Andreas S (2004) Classic toxin-induced animal models of Parkinson’s disease: 6-OHDA and MPTP. Cell Tissue Res 318:215–224
Potashkin JA, Blume SR, Runkle NK (2011) Limitations of animal models of Parkinson’s. Parkinsons Dis 2011:1–7
Yadav S, Dixit A, Agrawal S, Singh A, Srivastava G, Singh AK, Srivastava PK, Prakash O, Singh MP (2012) Rodent models and contemporary molecular techniques: notable feats yet incomplete explanations of Parkinson’s disease pathogenesis. Mol Neurobiol 829:1–8
Somayajulu-Niţu M, Sandhu JK, Cohen J, Sikorska M, Sridhar TS, Matei A, Borowy-Borowski H, Pandey S (2009) Paraquat induces oxidative stress, neuronal loss in substantia nigra region and parkinsonism in adult rats: neuroprotection and amelioration of symptoms by water soluble formulation of coenzyme Q10. BMC Neurosci 10:1–12
Zhang J, Fitsanakis VA, Gu G, Jing D, Ao M, Amarnath V, Montine TJ (2003) Manganese ethylene-bis-dithiocarbamate and selective dopaminergic neurodegeneration in rat: a link through mitochondrial dysfunction. J Neuro-chem 84:336–346
Brooks AI, Chadwick CA, Gelbard HA, Cory-Slechta DA, Federoff HJ (1999) Paraquat elicited neurobehavioral syndrome caused by dopaminergic neuron loss. Brain Res 823:1–10
Thiruchelvam M, Richfield EK, Baggs RB, Tank AW, Cory-Slechta DA (2000) The nigrostriatal dopaminergic system as a preferential target of repeated exposures to combined paraquat and maneb: implica-tions for Parkinson’s disease. J Neurosci 20:9207–9214
Gupta SP, Patel S, Yadav S, Singh AK, Singh S, Singh MP (2010) Involvement of nitric oxide in maneb and paraquat-induced Parkinson’s disease phenotype in mouse: is there any link with lipid peroxidation? Neurochem Res 35:1206–1213
Mishra LC, Singh BB, Dagenais S (2000) Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Altern Med Rev 5:334–346
Sadrzadeh SMH, Saffari Y (2004) Iron and brain disorders. Am J Clin Pathol 121:64–70
Halliwell B, Gutteridge JMC (1985) Oxygen radicals and the nervous system. Trends Neurosci 8:22–29
Kale M, Rathore N, John S, Bhatnagar D (1999) Lipid peroxidative damage on pyrethroid exposure and alterations in antioxidant status in rat erythrocytes: a possible involvement of reactive oxygen species. Toxicol Lett 105:197–205
Nagashayana N, Sankarankutty P, Nampoothiri MR, Mohan PK, Mohanakumar KP (2000) Association of L-DOPA with recovery following ayurveda medication in Parkinson’s disease. J Neurol Sci 176:124–127
Konar A, Shah N, Singh R, Saxena N, Kaul SC, Wadhwa R, Thakur MK (2011) Protective role of Ashwagandha leaf extract and its component with an one on scopolamine-induced changes in the brain and brain-derived cells. PLoS One 6:1–11
Koppula S, Kumar H, More SV, Kim BW, Kim IS, Choi DK (2012) Recent advances on the neuroprotective potential of antioxidants in experimental models of Parkinson’s disease. Int J Mol Sci 13:10608–10629
Bhattacharya A, Ghosal S, Bhattacharya SK (2001) Anti-oxidant effect of Withania somnifera glycowithanolides in chronic footshock stress-induced perturbations of oxidative free radical scavenging enzymes and lipid peroxidation in rat frontal cortex and striatum. J Ethnopharmacol 74:1–6
Grandhi A, Mujumdar AM, Patwardhan B (1994) A comparative pharmacological investigation of Ashwagandha and Ginseng. J Ethnopharmacol 44:13–15
Kulkarni SK, Dhir A (2008) Withania somnifera: an Indian ginseng. Prog Neuro Pharmacol Biol Psychiatry 32:1093–1105
Kuboyama T, Tohda C, Zhao J, Nakamura N, Hattori M, Komatsu K (2002) Axon or dendrite predominant outgrowth induced by constituents from Ashwagandha. Neuro Rep 13:1715–1720
Tohda C, Kuboyama T, Komatsu K (2005) Search for natural products related to regeneration of the neuronal network. Neurosignals 14:34–45
Rajasankar S, Manivasagam T, Surendran S (2009) Ashwagandha leaf extract: a potential agent in treating oxidative damage and physiological abnormalities seen in a mouse model of Parkinson’s disease. Neurosci Lett 454:11–15
Rajasankara S, Manivasagam T, Sankar V, Prakash S, Muthusamy R, Krishnamurti A, Surendran S (2009) Withania somnifera root extract improves catecholamines and physiological abnormalities seen in a Parkinson’s disease model mouse. J Ethnopharmacol 125:369–373
Surendran S, Shihabuddin LS, Clarke J, Taksir TV, Stewart GR, Parsons G, Yang W, Tyring SK, Michals-Matalon K, Matalon R (2004) Mouse neural progenitor cells differentiate into oligodendrocytes in the brain of a knockout mouse model of Canavan disease. Brain Res Dev Brain Res 153:19–27
Mohanasundari M, Srinivasan MS, Sethupathy S, Sabesan M (2006) Enhanced neuroprotective effect by combination of bromocriptine and Hypericum perforatum extract against MPTP-induced neurotoxicity in mice. J Neurol Sci 249:140–144
Pisa M (1998) Regional specialization of motor functions in the rat striatum: implications for the treatment of Parkinsonism. Prog Neuro Pharmacol Biol Psychiatry 12:217–224
Tillerson JL, Miller GW (2003) Grid performance test to measure behavioral impairment in the MPTP-treated-mouse model of parkinsonism. J Neurosci Methods 123:189–200
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Granger DL, Taintor RR, Boockvar KS, Hibbs JB (1996) Measurement of nitrate and nitrite in biological samples using nitrate reductase and Greiss reaction. Methods Enzymol 268:142–151
Sinha AK (1972) Colorimetric assay of catalase. Anal Chem 47:389–394
Kumar A, Ahmad I, Shukla S, Singh BK, Patel DK, Pandey HP, Singh C (2010) Effect of zinc and paraquat co-exposure on neurodegeneration: modulation of oxidative stress and expression of metallothioneins, toxicant responsive and transporter genes in rats. Free Radic Res 44:950–965
Singh AK, Tiwari MN, Upadhyay G, Patel DK, Singh D, Prakash O, Singh MP (2012) Long-term exposure to cypermethrin induces nigrostriatal dopaminergic neurodegeneration in adult rats: postnatal exposure enhances the susceptibility during adulthood. Neurobiol Aging 33:404–415
Mochizuki H, Hayakawa H, Migita M, Shibata M, Tanaka R, Suzuki A, Shimo-Nakanishi Y, Urabe T, Yamada M, Tamayose K, Shimada T, Miura M, Mizuno Y (2001) An AAV-derived Apaf-1 dominant negative inhibitor prevents MPTP toxicity as antiapoptotic gene therapy for Parkinson’s disease. Proc Natl Acad Sci USA 98:10918–10923
Kidd PM (2000) Parkinson’s disease as multifactorial oxidative neurodegeneration: implications for integrative management. Altern Med Rev 6:502–529
Rajasankar S, Manivasagam T, Albert Singh T, Krishnamurti A, Ramanathan M (2007) Prophylactic effect of Withania somonifera against MPTP induced Parkin-son’s disease in mice. Cell Tissue Res 7:975–979
Schwarting RK, Bonatz AE, Carey RJ, Huston JP (1991) Relationships between indices of behavioral asymmetries and neurochemical changes following mesencephalic 6-hydroxydopamine injections. Brain Res 554:46–55
Fernagut PO, Diguet E, Labattu B, Tison F (2002) A simple method to measure stride length as an index of nigrostriatal dysfunction in mice. J Neurosci Methods 113:123–130
Zafar KS, Sayeed I, Siddiqui A, Ahmad M, Salim S, Islam F (2003) Dose-dependent protective effect of selenium in partial lesion model of Parkinson’s disease: neurobehavioral and neurochemical evidences. J Neurochem 84:438–446
Ahmad M, Yousuf S, Khan MB, Saleem S, Ishrat T, Hoda Md N, Islam F (2006) Protective effects of ethanolic extract of delphinium denudatumin a rat model of Parkinson’s disease. Human Expt Toxicol 25:361–368
Dexter DT, Wells FR, Lees AJ, Agid F, Agid Y, Jenner P, Marsden CD (1989) Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson’s disease. J Neurochem 52:1830–1836
Noack H, Possel H, Rethfeldt C, Keilhoff G, Wolf G (1999) Peroxynitrite med-iated damage and lowered superoxide tolerance in primary cor-tical glial cultures after induction of the inducible isoform of NOS. Glia 28:13–24
Denicola A, Radi R (2005) Peroxynitrite and drug-dependent toxicity. Toxicology 208:273–288
Antunes F, Nunes C, Laranjinha J, Cadenas E (2005) Redox interactions of nitric oxide with dopamine and its derivatives. Toxicology 208:207–212
Jagetia GC, Malagi KJ, Baliga MS, Venkatesh P, Veruva RR (2004) Triphala, an ayurvedic rasayana drug, protects mice against radiation-induced lethality by free-radical scavenging. J Altern Complement Med 10:971–978
Bhatnagar M, Sharma D, Salvi M (2009) Neuroprotective effects of Withania somnifera dunal.: a possible mechanism. Neurochem Res 34:1975–1983
Ahmad I, Kumar A, Shukla S, Prasad Pandey H, Singh C (2008) The involvement of nitric oxide in maneb- and paraquat-induced oxidative stress in rat polymorphonuclear leukocytes. Free Radic Res 42:849–862
Yadav S, Gupta SP, Srivastava G, Srivastava PK, Singh MP (2011) Role of secondary mediators in caffeine-mediated neuroprotection in maneb and paraquat induced Parkinson’s disease phenotype in the mouse. Neurochem Res 37:875–884
Cheng Y, He G, Mu X, Zhang T, Li X, Hu J, Xu B, Du G (2008) Neuroprotective effect of baicalein against MPTP neurotoxicity: behavioral, biochemical and immunohistochemical profile. Neurosci Lett 441:16–20
Acknowledgments
Authors are thankful to Dr Andrew Wolfe, Assistant Professor, Department of Pediatric Endocrinology of Johns Hopkins University, Baltimore, Maryland, USA, Miss Samantha Leahy, Department of Chemistry and Biochemistry University of Windsor, Windsor, Canada and Dr. Ramesh Raina, Chair, Department of Biology, Syracuse University, Syracuse, NY, USA for their constructive suggestions while writing the paper. Authors wish to acknowledge Dr. T.D. Singh, Associate Professor, Department of Medicinal Chemistry, IMS, BHU, for helping us to prepare ethanolic root extract of Ws in his laboratory. The authors sincerely thank Indian council of Medical research (ICMR), New Delhi, India for providing research fellowships to Jay Prakash, University Grant Commission, New Delhi, India for providing fellowship to Shikha Chouhan and Council of Scientific and Industrial Research (CSIR), New Delhi, India for providing research fellowship to Satyndra Kumar Yadav. The study was supported financially by Department of Science and Technology (DST P-07-520) New Delhi, India. The authors report no conflicts of interest and they themselves are responsible for the content and writing of the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prakash, J., Yadav, S.K., Chouhan, S. et al. Neuroprotective Role of Withania somnifera Root Extract in Maneb–Paraquat Induced Mouse Model of Parkinsonism. Neurochem Res 38, 972–980 (2013). https://doi.org/10.1007/s11064-013-1005-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-013-1005-4