Abstract
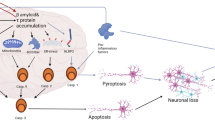
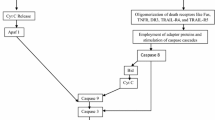
Apoptosis is an intrinsic biochemical, cellular process that regulates cell death and is crucial for cell survival, cellular homeostasis, and maintaining the optimum functional status. Apoptosis in a predetermined and programmed manner regulates several molecular events, including cell turnover, embryonic development, and immune system functions but may be the exclusive contributor to several disorders, including neurodegenerative manifestations, when it functions in an aberrant and disorganized manner. Alzheimer’s disease (AD) is a fatal, chronic neurodegenerative disorder where apoptosis has a compelling and divergent role. The well-characterized pathological features of AD, including extracellular plaques of amyloid-beta, intracellular hyperphosphorylated tangles of tau protein (NFTs), inflammation, mitochondrial dysfunction, oxidative stress, and excitotoxic cell death, also instigate an abnormal apoptotic cascade in susceptible brain regions (cerebral cortex, hippocampus). The apoptotic players in these regions affect cellular organelles (mitochondria and endoplasmic reticulum), interact with trophic factors, and several pathways, including PI3K/AKT, JNK, MAPK, mTOR signalling. This dysregulated apoptotic cascade end with an abnormal neuronal loss which is a primary event that may precede the other events of AD progression and correlates well with the degree of dementia. The present review provides insight into the diverse and versatile apoptotic mechanisms that are indispensable for neuronal survival and constitute an integral part of the pathological progression of AD. Identification of potential targets (restoring apoptotic and antiapoptotic balance, caspases, TRADD, RIPK1, FADD, TNFα, etc.) may be valuable and advantageous to decide the fate of neurons and to develop potential therapeutics for treatment of AD.





Similar content being viewed by others
Abbreviations
- AIF:
-
Apoptosis-Inducing Factor
- AMPK:
-
AMP-Activated Protein Kinase
- Apaf1:
-
Apoptotic Protease Activating Factor 1
- APP:
-
βAmyloid Precursor Protein
- ATF-6:
-
Activating Transcription Factor-6
- BAX:
-
Bcl-2 Associated X Protein
- CREB:
-
cAMP Response Element-Binding Protein
- ERK:
-
Extracellular Signalregulated Protein Kinase
- FADD:
-
Fas Associated Death Domain
- FKH/FOXO:
-
factor Forkhead/Forkhead Box Transcription Factors
- GSK3B:
-
Glycogen Synthase Kinase 3β
- IFN-γ:
-
Interferon-Gamma
- IRE1:
-
Inositol-Requiring Enzyme-1
- JAK:
-
Janus Kinase
- JNK:
-
c-Jun N-Terminal Kinase
- MAPK:
-
A Mitogen-Activated Protein Kinase
- mTOR:
-
Mammalian Target of Rapamycin
- NFKB:
-
Nuclear Factor Kappa B
- PARP1:
-
Poly (ADP-Ribose) Polymerase
- PERK:
-
Pancreatic ER Kinase
- PI3K:
-
Phosphatidylinositol-3-Kinase
- RIP:
-
Receptor Interacting Protein
- STAT:
-
Signal Transducer and Activator of Transcription
- TACE:
-
TNF-α Converting Enzyme
- TAT–TIJIP:
-
Tat Cell Transporter Sequence–Truncated Inhibitor of JNK Interacting Protein
- TNF-α:
-
Tumor Necrosis Factor Alpha
- TRADD:
-
TNF Receptor Associated Death Domain
- TSC1:
-
Tuberous Sclerosis Protein 1
- WNT:
-
Wingless-Related Integration
References
Bhute S, Sarmah D, Datta A, Rane P, Shard A, Goswami A, Borah A, Kalia K, Dave KR, Bhattacharya P (2020) Molecular pathogenesis and interventional strategies for Alzheimer’s disease: promises and pitfalls. ACS Pharmacol Transl Sci 3(3):472–488. https://doi.org/10.1021/acsptsci.9b00104
Sharma VK, Mehta V, Singh TG (2020) Alzheimer’s disorder: epigenetic connection and associated risk factors. Curr Neuropharmacol 18(8):740–753. https://doi.org/10.2174/1570159X18666200128125641
Sharma VK, Singh TG (2020) Navigating Alzheimer’s disease via chronic stress: the role of glucocorticoids. Curr Drug Targets 21(5):433–444. https://doi.org/10.2174/1389450120666191017114735
Su JH, Anderson AJ, Cummings BJ, Cotman CW (1994) Immunohistochemical evidence for apoptosis in Alzheimer’s disease. Neuroreport 5(18):2529–2533. https://doi.org/10.1097/00001756-199412000-00031
Sharma VK, Singh TG, Mehta V (2021) Stressed mitochondria: a target to intrude Alzheimer’s disease. Mitochondrion 59:48–57. https://doi.org/10.1016/j.mito.2021.04.004
Felderhoff-Mueser U, Sifringer M, Pesditschek S, Kuckuck H, Moysich A, Bittigau P, Ikonomidou C (2002) Pathways leading to apoptotic neurodegeneration following trauma to the developing rat brain. Neurobiol Dis 11(2):231–245. https://doi.org/10.1006/nbdi.2002.0521
Nisticò R, Borg JJ (2021) Aducanumab for Alzheimer’s disease: a regulatory perspective. Pharmacol Res 171:105754
Shimohama S (2000) Apoptosis in Alzheimer’s disease–an update. Apoptosis 5(1):9–16. https://doi.org/10.1023/a:1009625323388
Sharma VK, Singh TG, Singh S (2020) Cyclic nucleotides signaling and phosphodiesterase inhibition: defying Alzheimer’s disease. Curr Drug Targets 21(13):1371–1384. https://doi.org/10.2174/1389450121666200727104728
Sharma VK, Singh TG, Garg N, Dhiman S, Gupta S, Rahman MH, Najda A, Walasek-Janusz M, Kamel M, Albadrani GM, Akhtar MF, Saleem A, Altyar AE, Abdel-Daim MM (2021) Dysbiosis and Alzheimer’s disease: a role for chronic stress? Biomolecules 11(5):678. https://doi.org/10.3390/biom11050678
Long JY, Chen JM, Liao YJ, Zhou YJ, Liang BY, Zhou Y (2020) Naringin provides neuroprotection in CCL2-induced cognition impairment by attenuating neuronal apoptosis in the hippocampus. Behav Brain Funct 16(1):4. https://doi.org/10.1186/s12993-020-00166-6
Obulesu M, Lakshmi MJ (2014) Apoptosis in Alzheimer’s disease: an understanding of the physiology, pathology and therapeutic avenues. Neurochem Res 39(12):2301–2312. https://doi.org/10.1007/s11064-014-1454-4
Turunc Bayrakdar E, Uyanikgil Y, Kanit L, Koylu E, Yalcin A (2014) Nicotinamide treatment reduces the levels of oxidative stress, apoptosis, and PARP-1 activity in Aβ(1–42)-induced rat model of Alzheimer’s disease. Free Radic Res 48(2):146–158. https://doi.org/10.3109/10715762.2013.857018
Gómez-Isla T, Price JL, McKeel DW Jr., Morris JC, Growdon JH, Hyman BT (1996) Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci 16(14):4491–4500. https://doi.org/10.1523/JNEUROSCI.16-14-04491.1996
Cotman CW (1998) Apoptosis decision cascades and neuronal degeneration in Alzheimer’s disease. Neurobiol Aging 19(1 Suppl):S29–S32. https://doi.org/10.1016/s0197-4580(98)00042-6
Martínez-Pinilla E, Ordóñez C, Del Valle E, Navarro A, Tolivia J (2016) Regional and gender study of neuronal density in brain during aging and in Alzheimer’s disease. Front Aging Neurosci 8:213. https://doi.org/10.3389/fnagi.2016.00213
Wang YL, Li JF, Wang YT, Xu CY, Hua LL, Yang XP, Geng S, Wang SS, Wang Z, Yin HL (2017) Curcumin reduces hippocampal neuron apoptosis and JNK-3 phosphorylation in rats with Aβ-induced Alzheimer’s disease: protecting spatial learning and memory. J Neurorestoratol 5:117–123
LeBlanc AC (2005) The role of apoptotic pathways in Alzheimer’s disease neurodegeneration and cell death. Curr Alzheimer Res 2(4):389–402. https://doi.org/10.2174/156720505774330573
Tang D, Kang R, Berghe TV et al (2019) The molecular machinery of regulated cell death. Cell Res 29:347–364
O’Brien MA, Kirby R (2008) Apoptosis: a review of pro-apoptotic and anti‐apoptotic pathways and dysregulation in disease. J Vet Emerg Crit Care 18(6):572–585
Jellinger KA (2001) Cell death mechanisms in neurodegeneration. J Cell Mol Med 5(1):1–17. https://doi.org/10.1111/j.1582-4934.2001.tb00134.x
Cotman CW, Anderson AJ (1995) A potential role for apoptosis in neurodegeneration and Alzheimer’s disease. Mol Neurobiol 10(1):19–45. https://doi.org/10.1007/BF02740836
Wyllie AH (1980) Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 284(5756):555–556. https://doi.org/10.1038/284555a0
Liu X, Zou H, Slaughter C, Wang X (1997) DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell 89(2):175–184. https://doi.org/10.1016/s0092-8674(00)80197-x
Crowley LC, Marfell BJ, Waterhouse NJ (2016) Detection of DNA fragmentation in apoptotic cells by TUNEL. Cold Spring Harb Protoc. https://doi.org/10.1101/pdb.prot087221
Satija S, Kaur H, Tambuwala MM, Sharma P, Vyas M, Khurana N, Sharma N, Bakshi HA, Charbe NB, Zacconi FC, Aljabali AA, Nammi S, Dureja H, Singh TG, Gupta G, Dhanjal DS, Dua K, Chellappan DK, Mehta M (2021) Hypoxia-inducible factor (HIF): fuel for cancer progression. Curr Mol Pharmacol. https://doi.org/10.2174/1874467214666210120154929
Wu CK, Thal L, Pizzo D, Hansen L, Masliah E, Geula C (2005) Apoptotic signals within the basal forebrain cholinergic neurons in Alzheimer’s disease. Exp Neurol 195(2):484–496. https://doi.org/10.1016/j.expneurol.2005.06.020
Wang X (2009) The antiapoptotic activity of melatonin in neurodegenerative diseases. CNS Neurosci Ther 15(4):345–357. https://doi.org/10.1111/j.1755-5949.2009.00105.x
Ramalho RM, Viana RJ, Low WC, Steer CJ, Rodrigues CM (2008) Bile acids and apoptosis modulation: an emerging role in experimental Alzheimer’s disease. Trends Mol Med 14(2):54–62. https://doi.org/10.1016/j.molmed.2007.12.001
Roth KA (2001) Caspases, apoptosis, and Alzheimer disease: causation, correlation, and confusion. J Neuropathol Exp Neurol 60(9):829–838. https://doi.org/10.1093/jnen/60.9.829
Cheng M, Tang Z, Liang D, Zeng F, Liu R, Lian Q, Wu H (2020) The effects of Porphyromonas gingivalis on the apoptosis of hippocampal cells in Sprague-Dawley rats and its underlying mechanisms. Int J Clin Exp Med 13(1):300–309
Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, Woodbury P, Growdon J, Cotman CW, Pfeiffer E, Schneider LS (1997) A. controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. New Eng J Med 336(17):1216–1222
Fontaine RH, Cases O, Lelièvre V, Mesplès B, Renauld JC, Loron G, Degos V, Dournaud P, Baud O, Gressens P (2008) IL-9/IL-9 receptor signaling selectively protects cortical neurons against developmental apoptosis. Cell Death Differ 15(10):1542–1552. https://doi.org/10.1038/cdd.2008.79
Hu X, Song C, Fang M, Li C (2018) Simvastatin inhibits the apoptosis of hippocampal cells in a mouse model of Alzheimer’s disease. Exp Ther Med 15(2):1795–1802. https://doi.org/10.3892/etm.2017.5620
Oceandy D, Amanda B, Ashari FY, Faizah Z, Azis MA, Stafford N (2019) The cross-talk between the TNF-α and RASSF-hippo signalling pathways. Int J Mol Sci 20(9):2346. https://doi.org/10.3390/ijms20092346
Busca A, Saxena M, Kryworuchko M, Kumar A (2009) Anti-apoptotic genes in the survival of monocytic cells during infection. Curr Genom 10(5):306–317. https://doi.org/10.2174/138920209788920967
Fulda S (2009) Tumor resistance to apoptosis. Int J Cancer 124(3):511–515. https://doi.org/10.1002/ijc.24064
Ziegler DS, Kung AL, Kieran MW (2008) Anti-apoptosis mechanisms in malignant gliomas. J Clin Oncol 26(3):493–500. https://doi.org/10.1200/JCO.2007.13.9717
Chen L, Xu B, Liu L, Luo Y, Yin J, Zhou H, Chen W, Shen T, Han X, Huang S (2010) Hydrogen peroxide inhibits mTOR signaling by activation of AMPKalpha leading to apoptosis of neuronal cells. Lab Invest 90(5):762–773. https://doi.org/10.1038/labinvest.2010.36
Pias EK, Aw TY (2002) Early redox imbalance mediates hydroperoxide-induced apoptosis in mitotic competent undifferentiated PC-12 cells. Cell Death Differ 9(9):1007–16. https://doi.org/10.1038/sj.cdd.4401064
Okouchi M, Ekshyyan O, Maracine M, Aw TY (2007) Neuronal apoptosis in neurodegeneration. Antioxid Redox Signal 9(8):1059–1096. https://doi.org/10.1089/ars.2007.1511
Grewal AK, Singh TG, Sharma D, Sharma V, Singh M, Rahman MH, Najda A, Walasek-Janusz M, Kamel M, Albadrani GM, Akhtar MF, Saleem A, Abdel-Daim MM (2021) Mechanistic insights and perspectives involved in neuroprotective action of quercetin. Biomed Pharmacother 140:111729. https://doi.org/10.1016/j.biopha.2021.111729
Mattson MP, Culmsee C, Yu ZF (2000) Apoptotic and antiapoptotic mechanisms in stroke. Cell Tissue Res 301(1):173–187. https://doi.org/10.1007/s004419900154
Tubeleviciute-Aydin A, Zhou L, Sharma G, Soni IV, Savinov SN, Hardy JA, LeBlanc AC (2018) Rare human caspase-6-R65W and caspase-6-G66R variants identify a novel regulatory region of caspase-6 activity. Sci Rep 8(1):4428. https://doi.org/10.1038/s41598-018-22283-z
Guo H, Pétrin D, Zhang Y, Bergeron C, Goodyer CG, LeBlanc AC (2006) Caspase-1 activation of caspase-6 in human apoptotic neurons. Cell Death Differ 13(2):285–292. https://doi.org/10.1038/sj.cdd.4401753
Simon DJ, Weimer RM, McLaughlin T, Kallop D, Stanger K, Yang J, O’Leary DD, Hannoush RN, Tessier-Lavigne M (2012) A caspase cascade regulating developmental axon degeneration. J Neurosci 32(49):17540–17553. https://doi.org/10.1523/JNEUROSCI.3012-12.2012
Angel A, Volkman R, Royal TG, Offen D (2020) Caspase-6 knockout in the 5xFAD model of Alzheimer’s disease reveals favorable outcome on memory and neurological hallmarks. Int J Mol Sci 21(3):1144. https://doi.org/10.3390/ijms21031144
Tible M, Mouton Liger F, Schmitt J, Giralt A, Farid K, Thomasseau S, Gourmaud S, Paquet C, Rondi Reig L, Meurs E, Girault JA, Hugon J (2019) PKR knockout in the 5xFAD model of Alzheimer’s disease reveals beneficial effects on spatial memory and brain lesions. Aging Cell 18(3):e12887. https://doi.org/10.1111/acel.12887
Sclip A, Arnaboldi A, Colombo I, Veglianese P, Colombo L, Messa M, Mancini S, Cimini S, Morelli F, Antoniou X, Welker E, Salmona M, Borsello T (2013) Soluble Aβ oligomer-induced synaptopathy: c-Jun N-terminal kinase’s role. J Mol Cell Biol 5(4):277–279. https://doi.org/10.1093/jmcb/mjt015
Yoon SO, Park DJ, Ryu JC, Ozer HG, Tep C, Shin YJ, Lim TH, Pastorino L, Kunwar AJ, Walton JC, Nagahara AH, Lu KP, Nelson RJ, Tuszynski MH, Huang K (2012) JNK3 perpetuates metabolic stress induced by Aβ peptides. Neuron 75(5):824–837. https://doi.org/10.1016/j.neuron.2012.06.024
Yao M, Nguyen TV, Pike CJ (2005) Beta-amyloid-induced neuronal apoptosis involves c-Jun N-terminal kinase-dependent downregulation of Bcl-w. J Neurosci 25(5):1149–1158. https://doi.org/10.1523/JNEUROSCI.4736-04.2005
Tang SC, Lathia JD, Selvaraj PK, Jo DG, Mughal MR, Cheng A, Siler DA, Markesbery WR, Arumugam TV, Mattson MP (2008) Toll-like receptor-4 mediates neuronal apoptosis induced by amyloid beta-peptide and the membrane lipid peroxidation product 4-hydroxynonenal. Exp Neurol 213(1):114–121. https://doi.org/10.1016/j.expneurol.2008.05.014
Yarza R, Vela S, Solas M, Ramirez MJ (2016) c-Jun N-terminal kinase (JNK) signaling as a therapeutic target for Alzheimer’s disease. Front Pharmacol 6:321. https://doi.org/10.3389/fphar.2015.00321
Cui J, Zhang M, Zhang YQ, Xu ZH (2007) JNK pathway: diseases and therapeutic potential. Acta Pharmacol Sin 5:601–608. https://doi.org/10.1111/j.1745-7254.2007.00579.x
Lutz C, Nimpf J, Jenny M, Boecklinger K, Enzinger C, Utermann G, Baier-Bitterlich G, Baier G (2002) Evidence of functional modulation of the MEKK/JNK/cJun signaling cascade by the low density lipoprotein receptor-related protein (LRP). J Biol Chem 277(45):43143–43151. https://doi.org/10.1074/jbc.M204426200
Dhanasekaran DN, Reddy EP (2008) JNK signaling in apoptosis. Oncogene 27(48):6245–6251. https://doi.org/10.1038/onc.2008.301
Lu Z, Xu S (2006) ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life Nov 58(11):621–631. https://doi.org/10.1080/15216540600957438
Subramaniam S, Zirrgiebel U, von B Und Halbach, Strelau O, Laliberté J, Kaplan C, Unsicker DR K (2004) ERK activation promotes neuronal degeneration predominantly through plasma membrane damage and independently of caspase-3. J Cell Biol 165(3):357–369. https://doi.org/10.1083/jcb.200403028
Gupta A, Shah K, Oza MJ, Behl T (2019) Reactivation of p53 gene by MDM2 inhibitors: a novel therapy for cancer treatment. Biomed Pharmacother 109:484–492. https://doi.org/10.1016/j.biopha.2018.10.155
Sharma VK, Singh TG (2020) Chronic stress and diabetes mellitus: interwoven pathologies. Curr Diabetes Rev 16(6):546–556. https://doi.org/10.2174/1573399815666191111152248
Levenga J, Wong H, Milstead RA, Keller BN, LaPlante LE, Hoeffer CA (2017) AKT isoforms have distinct hippocampal expression and roles in synaptic plasticity. Elife 6:e30640. https://doi.org/10.7554/eLife.30640
Noguchi M, Suizu F (2012) Regulation of AKT by phosphorylation of distinct threonine and serine residues. Adv Med Biol 47:139–162
Sharma VK, Singh TG (2020) Insulin resistance and bioenergetic manifestations: targets and approaches in Alzheimer’s disease. Life Sci 262:118401. https://doi.org/10.1016/j.lfs.2020.118401
Kim R (2005) Unknotting the roles of Bcl-2 and Bcl-xL in cell death. Biochem Biophys Res Commun 333(2):336–343. https://doi.org/10.1016/j.bbrc.2005.04.161
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME (1999) Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96(6):857–868. https://doi.org/10.1016/s0092-8674(00)80595-4
Alberghina L, Colangelo AM (2006) The modular systems biology approach to investigate the control of apoptosis in Alzheimer’s disease neurodegeneration. BMC Neurosci 7(Suppl 1):S2. https://doi.org/10.1186/1471-2202-7-S1-S2. Suppl 1 ) .
Sharma V, Kaur A, Singh TG (2020) Counteracting role of nuclear factor erythroid 2-related factor 2 pathway in Alzheimer’s disease. Biomed Pharmacother 129:110373. https://doi.org/10.1016/j.biopha.2020.110373
Bhaskar K, Miller M, Chludzinski A, Herrup K, Zagorski M, Lamb BT (2009) The PI3K-Akt-mTOR pathway regulates Abeta oligomer induced neuronal cell cycle events. Mol Neurodegener 4:14. https://doi.org/10.1186/1750-1326-4-14
Khan H, Singh A, Thapa K, Garg N, Grewal AK, Singh TG (2021) Therapeutic modulation of the phosphatidylinositol 3-kinases (PI3K) pathway in cerebral ischemic injury. Brain Res 2:147399. https://doi.org/10.1016/j.brainres.2021.147399
Tramutola A, Triplett JC, Di Domenico F, Niedowicz DM, Murphy MP, Coccia R, Perluigi M, Butterfield DA (2015) Alteration of mTOR signaling occurs early in the progression of Alzheimer disease (AD): analysis of brain from subjects with pre-clinical AD, amnestic mild cognitive impairment and late-stage AD. J Neurochem 133(5):739–749. https://doi.org/10.1111/jnc.13037
Slee EA, Adrain C, Martin SJ (1999) Serial killers: ordering caspase activation events in apoptosis. Cell Death Differ 11:1067–1074. https://doi.org/10.1038/sj.cdd.4400601
Diez H, Garrido JJ, Wandosell F (2012) Specific roles of Akt iso forms in apoptosis and axon growth regulation in neurons. PLoS One 7(4):e32715. https://doi.org/10.1371/journal.pone.0032715
O’ Neill C (2013) PI3-kinase/Akt/mTOR signaling: impaired on/off switches in aging, cognitive decline and Alzheimer’s disease. Exp Gerontol 48(7):647–653
Kim DI, Lee KH, Gabr AA, Choi GE, Kim JS, Ko SH, Han HJ (2016) Aβ-Induced Drp1 phosphorylation through Akt activation promotes excessive mitochondrial fission leading to neuronal apoptosis. Biochim Biophys Acta 1863(11):2820–2834. https://doi.org/10.1016/j.bbamcr.2016.09.003
Hetman M, Xia Z (2000) Signaling pathways mediating anti-apoptotic action of neurotrophins. Acta Neurobiol Exp (Wars) 60(4):531–545
Grewal SS, York RD, Stork PJ (1999) Extracellular-signal-regulated kinase signalling in neurons. Curr Opin Neurobiol 9(5):544–553 (10.1016/S0959-4388(99)00010 – 0)
Franke TF, Kaplan DR, Cantley LC (1997) PI3K: downstream AKTion blocks apoptosis. Cell 88(4):435–437. https://doi.org/10.1016/s0092-8674(00)81883-8
Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME (1995) Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270(5240):1326–1331. https://doi.org/10.1126/science.270.5240.1326
Yao R, Cooper GM (1995) Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science 267(5206):2003–2006. https://doi.org/10.1126/science.7701324
Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER (1996) Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron 16(6):1137–1145. https://doi.org/10.1016/s0896-6273(00)80140-3
Yamaguchi A, Tamatani M, Matsuzaki H, Namikawa K, Kiyama H, Vitek MP, Mitsuda N, Tohyama M (2001) Akt activation protects hippocampal neurons from apoptosis by inhibiting transcriptional activity of p53. J Biol Chem 276(7):5256–5264. https://doi.org/10.1074/jbc.M008552200
Friedman WJ (2000) Neurotrophins induce death of hippocampal neurons via the p75 receptor. J Neurosci 20(17):6340–6346. https://doi.org/10.1523/JNEUROSCI.20-17-06340.2000
Gilman CP, Mattson MP (2002) Do apoptotic mechanisms regulate synaptic plasticity and growth-cone motility? Neuromolecular Med 2(2):197–214. https://doi.org/10.1385/NMM:2:2:197
Maiese K, Chong ZZ, Wang S, Shang YC (2012) Oxidant stress and signal transduction in the nervous system with the PI 3-K, Akt, and mTOR cascade. Int J Mol Sci 13(11):13830–13866. https://doi.org/10.3390/ijms131113830
Chen L, Xu B, Liu L, Luo Y, Yin J, Zhou H, Chen W, Shen T, Han X, Huang S (2010) Hydrogen peroxide inhibits mTOR signaling by activation of AMPK alpha leading to apoptosis of neuronal cells. Lab Invest 90(5):762–773. https://doi.org/10.1038/labinvest.2010.36
Chen C, Liu Y, Liu Y, Zheng P (2009) mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal 2(98):ra75. https://doi.org/10.1126/scisignal.2000559
Maiese K (2014) Driving neural regeneration through the mammalian target of rapamycin. Neural Regen Res 9(15):1413–1417. https://doi.org/10.4103/1673-5374.139453
Shang YC, Chong ZZ, Wang S, Maiese K (2013) Tuberous sclerosis protein 2 (TSC2) modulates CCN4 cytoprotection during apoptotic amyloid toxicity in microglia. Curr Neurovasc Res 10(1):29–38. https://doi.org/10.2174/156720213804806007
Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, Carr SA, Sabatini DM (2007) PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell 25(6):903–915. https://doi.org/10.1016/j.molcel.2007.03.003
Sharma T, Kaur D, Grewal AK, Singh TG (2021) Therapies modulating insulin resistance in Parkinson’s disease: a cross talk. Neurosci Lett 749:135754. https://doi.org/10.1016/j.neulet.2021.135754
Huang J, Manning BD (2008) The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem J 412(2):179–190. https://doi.org/10.1042/BJ20080281
Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, Wang CY, He X, MacDougald OA, You M, Williams BO, Guan KL (2006) TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126(5):955–968. https://doi.org/10.1016/j.cell.2006.06.055
Inoki K, Zhu T, Guan KL (2003) TSC2 mediates cellular energy response to control cell growth and survival. Cell 115(5):577–590. https://doi.org/10.1016/s0092-8674(03)00929-2
Hardie DG (2007) AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol 8(10):774–785. https://doi.org/10.1038/nrm2249
Kimura R, Okouchi M, Fujioka H, Ichiyanagi A, Ryuge F, Mizuno T, Imaeda K, Okayama N, Kamiya Y, Asai K, Joh T (2009) Glucagon-like peptide-1 (GLP-1) protects against methylglyoxal-induced PC12 cell apoptosis through the PI3K/Akt/mTOR/GCLc/redox signaling pathway. Neuroscience 162(4):1212–1219. https://doi.org/10.1016/j.neuroscience.2009.05.025
Lee DF, Kuo HP, Chen CT, Hsu JM, Chou CK, Wei Y, Sun HL, Li LY, Ping B, Huang WC, He X, Hung JY, Lai CC, Ding Q, Su JL, Yang JY, Sahin AA, Hortobagyi GN, Tsai FJ, Tsai CH, Hung MC (2007) IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell 130(3):440–455. https://doi.org/10.1016/j.cell.2007.05.058
Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB (2005) AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell 18(3):283–293. https://doi.org/10.1016/j.molcel.2005.03.027
Hung CM, Garcia-Haro L, Sparks CA, Guertin DA (2012) mTOR-dependent cell survival mechanisms. Cold Spring Harb Perspect Biol 4(12):a008771. https://doi.org/10.1101/cshperspect.a008771
Yates SC, Zafar A, Hubbard P, Nagy S, Durant S, Bicknell R, Wilcock G, Christie S, Esiri MM, Smith AD, Nagy Z (2013) Dysfunction of the mTOR pathway is a risk factor for Alzheimer’s disease. Acta Neuropathol Commun 1:3. https://doi.org/10.1186/2051-5960-1-3
Nicolas CS, Amici M, Bortolotto ZA, Doherty A, Csaba Z, Fafouri A, Dournaud P, Gressens P, Collingridge GL, Peineau S (2013) The role of JAK-STAT signaling within the CNS. JAKSTAT 2(1):e22925. https://doi.org/10.4161/jkst.22925
Mäkelä J, Koivuniemi R, Korhonen L, Lindholm D (2010) Interferon-gamma produced by microglia and the neuropeptide PACAP have opposite effects on the viability of neural progenitor cells. PLoS One 5(6):e11091
Sharma VK, Singh TG (2020) CREB: a multifaceted target for Alzheimer’s disease. Curr Alzheimer Res 17(14):1280–1293. https://doi.org/10.2174/1567205018666210218152253
Pugazhenthi S, Wang M, Pham S, Sze CI, Eckman CB (2011) Downregulation of CREB expression in Alzheimer’s brain and in Aβ-treated rat hippocampal neurons. Mol Neurodegener 6:60. https://doi.org/10.1186/1750-1326-6-60
Che H, Zhang L, Ding L, Xie W, Jiang X, Xue C, Zhang T, Wang Y (2020) EPA-enriched ethanolamine plasmalogen and EPA-enriched phosphatidylethanolamine enhance BDNF/TrkB/CREB signaling and inhibit neuronal apoptosis in vitro and in vivo. Food Funct 11(2):1729–1739. https://doi.org/10.1039/c9fo02323b
Pugazhenthi S, Nesterova A, Jambal P, Audesirk G, Kern M, Cabell L, Eves E, Rosner MR, Boxer LM, Reusch JE (2003) Oxidative stress-mediated down-regulation of bcl-2 promoter in hippocampal neurons. J Neurochem 84(5):982–996. https://doi.org/10.1046/j.1471-4159.2003.01606.x
Sedger LM, McDermott MF (2014) TNF and TNF-receptors: from mediators of cell death and inflammation to therapeutic giants—past, present and future. Cytokine Growth Factor Rev 25(4):453–472. https://doi.org/10.1016/j.cytogfr.2014.07.016
Kalliolias GD, Ivashkiv LB (2016) TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol 12(1):49–62. https://doi.org/10.1038/nrrheum.2015.169
Brenner D, Blaser H, Mak TW (2015) Regulation of tumour necrosis factor signalling: live or let die. Nat Rev Immunol 15(6):362–374. https://doi.org/10.1038/nri3834
Xu X, Lai Y, Hua ZC (2019) Apoptosis and apoptotic body: disease message and therapeutic target potentials. Biosci Rep 39(1):BSR20180992. https://doi.org/10.1042/BSR20180992
Zang G, Fang L, Chen L, Wang C (2018) Ameliorative effect of nicergoline on cognitive function through the PI3K/AKT signaling pathway in mouse models of Alzheimer’s disease. Mol Med Rep 17(5):7293–7300. https://doi.org/10.3892/mmr.2018.8786
Singh S, Singh TG (2020) Role of nuclear factor kappa B (NF-κB) signalling in neurodegenerative diseases: an mechanistic approach. Curr Neuropharmacol 18(10):918–935. https://doi.org/10.2174/1570159X18666200207120949
Miranda S, Opazo C, Larrondo LF, Muñoz FJ, Ruiz F, Leighton F, Inestrosa NC (2000) The role of oxidative stress in the toxicity induced by amyloid beta-peptide in Alzheimer’s disease. Prog Neurobiol 62(6):633–648. https://doi.org/10.1016/s0301-0082(00)00015-0
Jha NK, Jha SK, Kar R, Nand P, Swati K, Goswami VK (2019) Nuclear factor-kappa β as a therapeutic target for Alzheimer’s disease. J Neurochem 150(2):113–137. https://doi.org/10.1111/jnc.14687
Kaur U, Banerjee P, Bir A, Sinha M, Biswas A, Chakrabarti S (2015) Reactive oxygen species, redox signaling and neuroinflammation in Alzheimer’s disease: the NF-κB connection. Curr Top Med Chem 15(5):446–457. https://doi.org/10.2174/1568026615666150114160543
Singh S, Singh TG, Rehni AK (2020) An insight into molecular mechanisms and novel therapeutic approaches in epileptogenesis. CNS Neurol Disord Drug Targets 19(10):750–779. https://doi.org/10.2174/1871527319666200910153827
Jones SV, Kounatidis I (2017) Nuclear factor-kappa B and Alzheimer disease, unifying genetic and environmental risk factors from cell to humans. Front Immunol 8:1805. https://doi.org/10.3389/fimmu.2017.01805
Degterev A, Ofengeim D, Yuan J (2019) Targeting RIPK1 for the treatment of human diseases. Proc Natl Acad Sci USA 116(20):9714–9722. https://doi.org/10.1073/pnas.1901179116
Barrier L, Fauconneau B, Noël A, Ingrand S (2010) Ceramide and related-sphingolipid levels are not altered in disease-associated brain regions of APP and APP/PS1 mouse models of Alzheimer’s disease: relationship with the lack of neurodegeneration? Int J Alzheimers Dis 2011:920958. https://doi.org/10.4061/2011/920958
Michikawa M (2003) The role of cholesterol in pathogenesis of Alzheimer’s disease: dual metabolic interaction between amyloid beta-protein and cholesterol. Mol Neurobiol 27(1):1–12. https://doi.org/10.1385/MN:27:1:1
Ledesma MD, Dotti CG (2006) Amyloid excess in Alzheimer’s disease: what is cholesterol to be blamed for? FEBS Lett 580(23):5525–5532. https://doi.org/10.1016/j.febslet.2006.06.038
van Kruining D, Luo Q, van Echten-Deckert G, Mielke MM, Bowman A, Ellis S, Oliveira TG, Martinez-Martinez P (2020) Sphingolipids as prognostic biomarkers of neurodegeneration, neuroinflammation, and psychiatric diseases and their emerging role in lipidomic investigation methods. Adv Drug Deliv Rev 159:232–244. https://doi.org/10.1016/j.addr.2020.04.009
Jazvinšćak Jembrek M, Hof PR, Šimić G (2015) Ceramides in Alzheimer’s disease: key mediators of neuronal apoptosis induced by oxidative stress and Aβ accumulation. Oxid Med Cell Longev 2015:346783. https://doi.org/10.1155/2015/346783
Czubowicz K, Jęśko H, Wencel P, Lukiw WJ, Strosznajder RP (2019) The role of ceramide and sphingosine-1-phosphate in Alzheimer’s disease and other neurodegenerative disorders. Mol Neurobiol 56(8):5436–5455. https://doi.org/10.1007/s12035-018-1448-3
Perez Ortiz JM, Swerdlow RH (2019) Mitochondrial dysfunction in Alzheimer’s disease: role in pathogenesis and novel therapeutic opportunities. Br J Pharmacol 176(18):3489–3507. https://doi.org/10.1111/bph.14585
Khan H, Gupta A, Singh TG, Kaur A (2021) Mechanistic insight on the role of leukotriene receptors in ischemic-reperfusion injury. Pharmacol Rep. https://doi.org/10.1007/s43440-021-00258-8
Hashimoto S, Saido TC (2018) Critical review: involvement of endoplasmic reticulum stress in the aetiology of Alzheimer’s disease. Open Biol 8(4):180024. https://doi.org/10.1098/rsob.180024
Gerakis Y, Hetz C (2018) Emerging roles of ER stress in the etiology and pathogenesis of Alzheimer’s disease. FEBS J 285(6):995–1011. https://doi.org/10.1111/febs.14332
Li JQ, Yu JT, Jiang T, Tan L (2015) Endoplasmic reticulum dysfunction in Alzheimer’s disease. Mol Neurobiol 51(1):383–395. https://doi.org/10.1007/s12035-014-8695-8
Huang HC, Tang D, Lu SY, Jiang ZF (2015) Endoplasmic reticulum stress as a novel neuronal mediator in Alzheimer’s disease. Neurol Res 37(4):366–374. https://doi.org/10.1179/1743132814Y.0000000448
Rahman MM, Shrestha L, Gulshan MA (2020) ER stress signaling in Alzheimer’s disease: molecular mechanisms and therapeutic implications. Quality control of cellular protein in neurodegenerative disorders. IGI Global, Hershey, pp 180–211
Plácido AI, Pereira CM, Duarte AI, Candeias E, Correia SC, Santos RX, Carvalho C, Cardoso S, Oliveira CR, Moreira PI (2014) The role of endoplasmic reticulum in amyloid precursor protein processing and trafficking: implications for Alzheimer’s disease. Biochim Biophys Acta 1842(9):1444–1453. https://doi.org/10.1016/j.bbadis.2014.05.003
Arrázola MS, Silva-Alvarez C, Inestrosa NC (2015) How the Wnt signaling pathway protects from neurodegeneration: the mitochondrial scenario. Front Cell Neurosci 9:166. https://doi.org/10.3389/fncel.2015.00166
Palomer E, Buechler J, Salinas PC (2019) Wnt signaling deregulation in the aging and Alzheimer’s brain. Front Cell Neurosci 13:227. https://doi.org/10.3389/fncel.2019.00227
McLeod F, Salinas PC (2018) Wnt proteins as modulators of synaptic plasticity. Curr Opin Neurobiol 53:90–95. https://doi.org/10.1016/j.conb.2018.06.003
Oliva CA, Vargas JY, Inestrosa NC (2013) Wnts in adult brain: from synaptic plasticity to cognitive deficiencies. Front Cell Neurosci 7:224. https://doi.org/10.3389/fncel.2013.00224
Rosso SB, Inestrosa NC (2013) WNT signaling in neuronal maturation and synaptogenesis. Front Cell Neurosci 7:103. https://doi.org/10.3389/fncel.2013.00103
Wan W, Xia S, Kalionis B, Liu L, Li Y (2014) The role of Wnt signaling in the development of Alzheimer’s disease: a potential therapeutic target? Biomed Res Int 2014:301575. https://doi.org/10.1155/2014/301575
Chen S, Guttridge DC, You Z, Zhang Z, Fribley A, Mayo MW, Kitajewski J, Wang CY (2001) Wnt-1 signaling inhibits apoptosis by activating beta-catenin/T cell factor-mediated transcription. J Cell Biol 152(1):87–96. https://doi.org/10.1083/jcb.152.1.87
Khan H, Tiwari P, Kaur A, Singh TG (2021) Sirtuin acetylation and deacetylation: a complex paradigm in neurodegenerative disease. Mol Neurobiol. https://doi.org/10.1007/s12035-021-02387-w
Love S, Barber R, Wilcock GK (1999) Increased poly(ADP-ribosyl)ation of nuclear proteins in Alzheimer’s disease. Brain 122(Pt 2):247–253. https://doi.org/10.1093/brain/122.2.247
Thapa K, Khan H, Sharma U, Grewal AK, Singh TG (2021) Poly (ADP-ribose) polymerase-1 as a promising drug target for neurodegenerative diseases. Life Sci 267:118975. https://doi.org/10.1016/j.lfs.2020.118975
Czapski GA, Cieślik M, Wencel PL, Wójtowicz S, Strosznajder RP, Strosznajder JB (2018) Inhibition of poly(ADP-ribose) polymerase-1 alters expression of mitochondria-related genes in PC12 cells: relevance to mitochondrial homeostasis in neurodegenerative disorders. Biochim Biophys Acta Mol Cell Res 1865(2):281–288. https://doi.org/10.1016/j.bbamcr.2017.11.003
De Smet C, Loriot A, Boon T (2004) Promoter-dependent mechanism leading to selective hypomethylation within the 5’ region of gene MAGE-A1 in tumor cells. Mol Cell Biol 24(11):4781–4790. https://doi.org/10.1128/MCB.24.11.4781-4790.2004
Wang SC, Oelze B, Schumacher A (2008) Age-specific epigenetic drift in late-onset Alzheimer’s disease. PLoS One 3(7):e2698. https://doi.org/10.1371/journal.pone.0002698
Wang J, Yu JT, Tan MS, Jiang T, Tan L (2013) Epigenetic mechanisms in Alzheimer’s disease: implications for pathogenesis and therapy. Ageing Res Rev 12(4):1024–1041. https://doi.org/10.1016/j.arr.2013.05.003
Millan MJ (2014) The epigenetic dimension of Alzheimer’s disease: causal, consequence, or curiosity? Dialogues Clin Neurosci 16(3):373–393. https://doi.org/10.31887/DCNS.2014.16.3/mmillan
Jackson-Grusby L, Beard C, Possemato R, Tudor M, Fambrough D, Csankovszki G, Dausman J, Lee P, Wilson C, Lander E, Jaenisch R (2001) Loss of genomic methylation causes p53-dependent apoptosis and epigenetic deregulation. Nat Genet 27(1):31–39. https://doi.org/10.1038/83730
Li P, Marshall L, Oh G, Jakubowski JL, Groot D, He Y, Wang T, Petronis A, Labrie V (2019) Epigenetic dysregulation of enhancers in neurons is associated with Alzheimer’s disease pathology and cognitive symptoms. Nat Commun 10(1):2246. https://doi.org/10.1038/s41467-019-10101-7
Lin L, Liu G, Yang L (2019) Crocin improves cognitive behavior in rats with Alzheimer’s disease by regulating endoplasmic reticulum stress and apoptosis. Biomed Res Int 2019:9454913. https://doi.org/10.1155/2019/9454913
Jin H, Wang M, Wang J, Cao H, Niu W, Du L (2020) Paeonol attenuates isoflurane anesthesia-induced hippocampal neurotoxicity via modulation of JNK/ERK/P38MAPK pathway and regulates histone acetylation in neonatal rat. J Matern Fetal Neonatal Med 33(1):81–91. https://doi.org/10.1080/14767058.2018.1487396
Chu Q, Zhu Y, Cao T, Zhang Y, Chang Z, Liu Y, Lu J, Zhang Y (2020) Studies on the neuroprotection of osthole on glutamate-induced apoptotic cells and an Alzheimer’s disease mouse model via modulation oxidative stress. Appl Biochem Biotechnol 190(2):634–644. https://doi.org/10.1007/s12010-019-03101-2
Kim YJ, Kim SH, Park Y, Park J, Lee JH, Kim BC, Song WK (2020) miR-16-5p is upregulated by amyloid β deposition in Alzheimer’s disease models and induces neuronal cell apoptosis through direct targeting and suppression of BCL-2. Exp Gerontol 136:110954. https://doi.org/10.1016/j.exger.2020.110954
Zhang R, Zheng Y, Hu F, Meng X, Lv B, Lao K, Gao X, Zhang X, Gou X (2020) Effect of (m)VD-hemopressin against Aβ1-42-induced oxidative stress and apoptosis in mouse hippocampal neurons. Peptides 124:170185. https://doi.org/10.1016/j.peptides.2019.170185
Ghamari F, Vaezi G, Khaksari M, Hojati V (2020) Nesfatin-1 ameliorate learning and memory deficit via inhibiting apoptosis and neuroinflammation following ethanol-induced neurotoxicity in early postnatal rats. Int. J. Pept. Res. Ther 26:2029
Hu X, Song C, Fang M, Li C (2017) Simvastatin inhibits the apoptosis of hippocampal cells in a mouse model of Alzheimer’s disease. Exp Ther Med 15(2):1795–1802. https://doi.org/10.3892/etm.2017.5620
Wang Y, Cai B, Shao J, Wang TT, Cai RZ, Ma CJ, Han T, Du J (2016) Genistein suppresses the mitochondrial apoptotic pathway in hippocampal neurons in rats with Alzheimer’s disease. Neural Regen Res 11(7):1153–1158. https://doi.org/10.4103/1673-5374.187056
Zhang D, Wang Z, Sheng C, Peng W, Hui S, Gong W, Chen S (2015) Icariin prevents amyloid beta-induced apoptosis via the PI3K/Akt pathway in PC-12 cells. Evid Based Complement Alternat Med 2015:235265. https://doi.org/10.1155/2015/235265
Gong Y, Chen J, Jin Y, Wang C, Zheng M, He L (2020) GW9508 ameliorates cognitive impairment via the cAMP-CREB and JNK pathways in APPswe/PS1dE9 mouse model of Alzheimer’s disease. Neuropharmacology 164:107899. https://doi.org/10.1016/j.neuropharm.2019.107899
Liu P, Cui L, Liu B, Liu W, Hayashi T, Mizuno K, Hattori S, Ushiki-Kaku Y, Onodera S, Ikejima T (2021) Silibinin ameliorates STZ-induced impairment of memory and learning by up- regulating insulin signaling pathway and attenuating apoptosis. Physiol Behav. https://doi.org/10.1016/j.physbeh.2019.112689
Szulwach KE, Jin P (2014) Integrating DNA methylation dynamics into a framework for understanding epigenetic codes. Bioessays 36(1):107–117. https://doi.org/10.1002/bies.201300090
Acknowledgements
The authors are grateful to the Chitkara College of Pharmacy, Chitkara University, Rajpura, Patiala, Punjab, India for providing the necessary facilities to carry out the research work.
Funding
Nil.
Author information
Authors and Affiliations
Contributions
Conceptualization: Conceived and designed the experiments: TGS. Analyzed the data: VKS, NG. Wrote the manuscript: VKS, SS. Visualization: VKS, TGS Editing of the Manuscript: VKS, SS, TGS Critically reviewed the article: TGS, SD. Supervision: TGS. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sharma, V.K., Singh, T.G., Singh, S. et al. Apoptotic Pathways and Alzheimer’s Disease: Probing Therapeutic Potential. Neurochem Res 46, 3103–3122 (2021). https://doi.org/10.1007/s11064-021-03418-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03418-7