Abstract
Proteins are responsible multiple biological functions, such as ligand binding, catalysis, and ion channeling. This functionality is enabled by proteins’ three-dimensional structures that require long polypeptides. Since plausibly prebiotic synthesis of functional polypeptides has proven challenging in the laboratory, we propose that these functions may have been initially performed by alternative macromolecular constructs, namely hyperbranched polymers (HBPs), during early stages of chemical evolution. HBPs can be straightforwardly synthesized in one-pot processes, possess globular structures determined by their architecture as opposed to folding in proteins, and have documented ligand binding and catalytic properties. Our initial study focuses on glycerol-citric acid HBPs synthesized via moderate heating in the dry state. The polymerization products consisted of a mixture of isomeric structures of varying molar mass as evidenced by NMR, mass spectrometry and size-exclusion chromatography. Addition of divalent cations during polymerization resulted in increased incorporation of citric acid into the HBPs and the possible formation of cation-oligomer complexes. The chelating properties of citric acid govern the makeup of the resulting polymer, turning the polymerization system into a rudimentary smart material.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biopolymers perform the majority of structural and catalytic functions in modern biology. Extensive research has been dedicated to the synthesis of modern biopolymers under prebiotically plausible conditions, either by direct condensation of α-amino acids and nucleotides under drying conditions (Fox and Harada 1958; Lahav et al. 1978), or in aqueous solution by employing activating reagents (Ferris et al. 1996; Liu and Orgel 1997; Schneider-Bernloehr et al. 1968; Schwendinger et al. 1995; Weber and Orgel 1978; Weimann et al. 1968). Without activating agents such syntheses require elevated temperature (250–300 °C) and low water activity due to the unfavorable thermodynamics of peptide and phosphodiester bond formation in aqueous solution (Martin 1998; Schwartz and Fox 1964). These extreme conditions, however, are often destructive to monomers and oligomers and, therefore, would prevent the formation of polymers. Furthermore, while biopolymer function is strongly dependent on the monomer sequence, current models using activated monomers lack robust sequence and structure control of the products, limiting the plausibility of functional polymer formation. We propose here an alternative model for the prebiotic synthesis of polymeric functional systems chemically different from those of contemporary biopolymers. These structures are capable of performing some of biopolymer functions, albeit in less efficient or selective fashion. Moreover, the utility of such structures is less dependent on size or monomer sequence than their biological counterparts. These readily synthesized macromolecules could have conceivably preceded biopolymers during the early stages of chemical evolution.
Proteins are biological macromolecules comprised of amino acids capable of folding into sequence specific three-dimensional structures that define their functions. The globular structure of proteins allows for the formation of cavities within them capable of specific ligand binding and modulation of the properties of the local environment, such as polarity and water activity. Consequently, the globular structure of protein is responsible for signaling, catalysis and ligand transport within a living organism. Protein function can be approximated by HBPs, a class of polymers with high degree of branching possessing a globular structure (Gao and Yan 2004; Kirkorian et al. 2012). The biomimetic catalytic activity of dendrimers, a subset polymer class of HBPs, is well documented (Astruc and Chardac 2001; Bhyrappa et al. 1996; Liu and Breslow 2003). HBPs have high propensity towards ligand binding either inside internal pockets with suitable environments or, alternatively, at the polymer surface that is characterized by a large number of potentially functional end groups (Kirkorian et al. 2012). The ligand binding capacity of HBPs makes them an attractive candidate for the development of drug delivery systems (Chen et al. 2008; Gao et al. 2003; Halpern et al. 2014; Hu et al. 2012; Kolhe et al. 2003; Prabaharan et al. 2009).
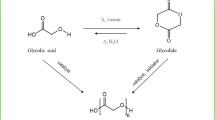
HBP systems based on condensation polymers are especially compelling in the context of prebiotic chemistry since all biomacromolecules are products of condensation dehydration polymerization. A number of condensation HBPs can be straightforwardly prepared in a one-pot system (Kienle et al. 1939; Tasaka et al. 2001; Wooley et al. 1994). HBP synthesis commonly involves condensation of monomers of ABx type, where A and B refer to different functional groups that can react with each other, due to their ready commercial availability. Flory’s statistical theory of mass distributions of three-dimensional polymers suggests that polymerization of monomers of ABx type can proceed infinitely without the occurrence of gelation due to the formation of crosslinked networks (Flory 1952). Condensation of ABx monomers has been used to prepare a wide range of polymeric products including polyphenylenes (Fréchet and Hawker 1995; Kim and Webster 1990) and polyesters (Wooley et al. 1994; Malmström et al. 1995; Malmström and Hult 1996). Alternatively, HBPs can be synthesized starting from homofunctional monomers as long as one of the functional groups is in stoichiometric excess (Gao and Yan 2004). Such systems, however, require a more rigorous control of monomer stoichiometry and degree of polymerization in order to avoid the formation of crosslinked networks; in some cases branching core inclusions are necessary to achieve hyperbranching (Malmström and Hult 1996). Our study concentrates on branched polyesters synthesized from A3 (glycerol) and AB3 (citric acid, Scheme 1) type monomers.
Esters are widespread in modern biochemistry mainly in the form of triesters of glycerol and fatty acids in lipids (Berg et al. 2012). An ester is the product of a condensation reaction. Ester formation has a slightly negative bond energy (~1 kcal/mol under physiological conditions (Williams et al. 1928), making this functional group’s formation facile. Polyesters have been hypothesized to have preceded peptides by Orgel (2003) due to their ease of formation, and this notion is perhaps supported by the demonstrated ability of the ribosome to catalyze α-hydroxy acid coupling (Fahnestock and Rich 1971; Fahnestock et al. 1970). Glyceric acid has been previously shown to polymerize and form polyester chains of up to 25 residues under acidic conditions and moderate heating. Polymers prepared from optically pure and racemic glyceric acid have different solubilities suggesting the possibility of chiral selection (Weber 1989). We have recently used malic acid polymerization as a model to investigate the far-from equilibrium conditions for prebiotic synthesis (Mamajanov et al. 2014).
Hyperbranched polyesters can be synthesized from a number of monomers found within a conceivable prebiotic inventory. For example, α-hydroxy acids are produced by spark-discharge experiments (Miller and Urey 1959). Additionally, glycolic and lactic acids along with α-hydroxyisovaleric and α-hydroxy-n-butyric have been identified in acid hydrolysates of samples obtained from Murchison meteorite (Peltzer and Bada 1978). Pizzarello et al. (Pizzarello et al. 2010) have detected a range of hydroxy acids in Murchison, GRA 95229 and LAP 02342 meteorites. Organic acids used to the Krebs cycle have been extensively studied in prebiotic context (Cody et al. 2001; Morowitz et al. 2000) and have also been detected in meteoritic samples (Cooper et al. 2011). A prebiotically plausible alcohol array includes carbohydrates presumed to be formed abiotically via formose (Breslow 1959; Ricardo et al. 2006) or glyoxylose (Sagi et al. 2012) processes and short polyols detected in the interstellar medium (Hollis et al. 2002) and in samples obtained from the Murchison and Murray meteorites (Cooper et al. 2001). Glycerol and glyceric acid have been detected in photolyzed astrophysical ice analogue residues (Bernstein et al. 2002). Sugar acids, bearing two necessary functional groups, are found in abundance as intermediates to carbohydrates in glyoxylose reactions (Butch et al. 2013; Sagi et al. 2012).
For this study we examined the condensation of glycerol and citric acid (Scheme 1). Both compounds are considered reasonably prebiotic. The physical properties, crosslinking content, and drug binding ability of glycerol-citric acid polymer have been previously described (Halpern et al. 2014). The polymers studied here were prepared in the dry state at temperatures varying from 90 to 130 °C at different starting monomer ratios; the degree of crosslinking depended on both the incubation temperature and monomer ratio. The goal of the present study was to investigate sequence, structure and heterogeneity of the glycerol-citric acid polymer synthesized at the lower end of the temperature range, examine the influence of divalent cations on the polyesterification process, and evaluate the polymer’s ability to bind divalent cations.
Materials and Methods
Citric acid (ACS reagent, Fisher Scientific), glycerol (99 %, Sigma-Aldrich) calcium chloride (1 M aqueous solution, Sigma-Aldrich), cobalt chloride hexahydrate (99.9 %, Alfa Aesar), copper chloride dihydrate (99.9 %, Alfa-Aesar), magnesium chloride hexahydrate (BioXtra, Sigma-Aldrich), nickel chloride (98 %, Alfa Aesar), zinc chloride (ACS reagent, Sigma-Aldrich) glycerol monoacetate (mixture of isomers, TCI America), glycerol diacetate (mixture of isomers, TCI America), and glycerol triacetate (98 %, Sigma-Aldrich) were used as received.
A typical polymerization reaction was conducted starting with 3 mL of an aqueous solution containing 330 mM citric acid, 330 or 660 mM glycerol, and 0-165 mM chloride salts as specified above. The pH of the solution was unadjusted, and was measured to be 2 before and after esterification suggesting that the polymer solutions were self-buffered by unreacted citric acid and incorporated free acid groups. The samples were incubated, uncovered, at 85 °C for 72 h until a constant dry weight was achieved.
1H and 13C Nuclear Magnetic Resonance (NMR) spectra were recorded on a Bruker DRX500 spectrometer at 25 °C. Spectra of polymer, monomers, mono-, di- and triacetin in D2O were used for assignments. 1H spectra were collected employing 30° inversion pulses with 11 s acquisition time and 1 s recycle delay. 13C NMR data was collected using inverse gated 30° inversion pulse with a 45 s recycle delay and proton decoupling during acquisition only to account for the T1 and minimize Nuclear Overhouser Effects (NOE). Chemical shift predictions were carried out using v.10.7 HNMR and CNMR packages of Advanced Chemistry Development (ACD, Toronto).
Mass spectrometry (MS) was performed using a Thermo Scientific LTQ Orbitrap XL mass spectrometer equipped with an electrospray ionization source operated in negative ion mode. The nitrogen sheath gas for desolvation of the electrospray was set to 3 (in arbitrary units) and the spray voltage was 3 kV. The tube lens was set to −155 V. Mass spectra were acquired from m/z 100 to 2000 using a mass resolution setting of 100,000 (full-width at half-maximum for m/z = 400). The mass spectrometer was calibrated using a mixture of caffeine, MRFA (L-methionyl–arginyl–phenylalanyl–alanine acetate hydrate) peptide, and Ultramark 1621 in an acetonitrile–methanol–water solution containing 1 % acetic acid. Reconstituted samples underwent a tenfold dilution in deionized nanofiltered water, followed by an additional 1:1 dilution in H2O:methanol with 0.3 % NH4OH.
For size exclusion chromatography–mass spectrometry (SEC-MS) analysis, the reconstituted dry samples were diluted 1:1 in 0.3 % aqueous solution of formic acid. The analysis was performed employing an Acquity Ultra Performance Liquid Chromatograph interfaced with a Triple Quadrupole (TQ) detector (Waters Corporation, Manchester, UK). The ionization was achieved by a corona discharge; sampling cone voltage was set at 15 V. The source and desolvation temperatures were set to 150 and 250 °C, respectively; the nitrogen desolvation flow rate was set to 500 L h−1. The mass spectrometer was calibrated from m/z 80–2000 using a 0.5 mM citric acid aqueous solution. The scan time was set to 0.5 s. The UPLC system used an Acquity APC column (125 Å, 2.5 μm, 4.6 mm × 30 mm, Waters Corp) column. An isocratic flow of 0.200 mL min−1 using H2O + 0.15 % formic acid was employed. The column temperature was set at 40 °C.
Results
Polyesterification
In order to approximate prebiotic conditions the polymerization in this study was performed by drying glycerol and citric acid solutions at specified ratios at 85 °C for 72 h without active water removal or catalyst addition except for systems containing divalent cation chlorides. Halpern et al. (2014) have shown that addition of p-toluene sulfonic acid and ZnCl2 during citric acid and glycerol polymerization had little impact on the physical properties of the resulting polymer. The polymerization of glycerol and citric acid at a 1:1 ratio resulted in the formation of partially water-insoluble product whereas the 2:1 ratio system resulted in fully soluble product and complete consumption of citric acid as indicated by 1H NMR as well as SEC-MS. This observation is consistent with Flory’s theory of gel formation in three dimensional polymers correlating the critical point for gelation with the degree of polymerization, as well as the number and reactivity of the monomer functional groups (Flory 1941a, b, c). The theory suggests that bifunctional polymerization systems with an excess of one of the functional groups will result in higher degrees of polymerization than systems with equal distribution of functions. To avoid gelation, our studies concentrated on glycerol: citric acid 2 : 1 systems. In fact, polymers synthesized at this molar ratio in the presence of divalent cation chlorides were soluble except for the preparations containing 0.5 equivalents of CuCl2 and ZnCl2 respectively relative to citric acid.
NMR
Figure 1 shows 1H NMR spectra in D2O of the products of the glycerol:citric acid 2:1 molar ratio polyesterification, neat and in the presence of the quarter equivalent of divalent cation chlorides relative to the citric acid, overlaid with unreacted starting materials. The spectrum of unreacted glycerol and citric acid (Fig. 1a) is characterized by the quadruplet at ~2.8 ppm corresponding to methylene hydrogens of citric acid, multiplets at ~3.4, 3.5 and 3.6 ppm representing methylene and methanetriyl hydrogens of glycerol. The spectrum of the neat preparation of the polyester (Fig. 1b) is described by broadened, downfield-shifted signals corresponding to citric acid unit methylene hydrogens, broadening of the glycerol unit hydrogens and the appearance of broad signals at ~4 and 5 ppm consistent with methylene and methanetryl hydrogens at the RCOO-CH position. The tentative signal assignments were performed with the assistance of ACD labs software predictions, 1H spectra of glycerol mono-, di- and tri-acetate (Fig. S1 and S2). The relative integrations of these signals suggests that only small fraction of the hydroxyls (>6 %) at the 2 position of glycerol are esterified possibly due to steric hindrance. The line broadening can be explained either by the presence of multiple oligomeric isomers with overlapping signals or, alternatively, by slowed molecular tumbling of polymeric species. Notably no residual citric acid signals are present in the neat preparation in contrast with the polymers prepared in the presence of CaCl2, MgCl2 and ZnCl2 as shown in Fig. 1c–e. Estimates based on integrated peak intensity suggest that 65, 74, and more than 95 % conversion is achieved in the presence of CaCl2, MgCl2 and ZnCl2, respectively. Conversion of glycerol based on the same analysis is estimated at 93, 65, 77, and 87 % for neat, CaCl2, MgCl2 and ZnCl2 systems, respectively.
Figure 2 depicts the downfield region containing carbonyl signals of 13C NMR spectra of the polyester samples described above. Quantitative 13C NMR data were collected using inverse gated 30° inversion pulse with a 45 s recycle delay and proton decoupling, during acquisition only, to account for the T1 and minimize NOE, respectively. In general, the polymers synthesized in the presence of MgCl2 and CaCl2 are characterized by sharper, better-resolved signals compared to the rest suggesting the presence of possibly shorter oligomers with uniform structure. The spectrum of unreacted starting materials is characterized by peaks at 176.6 and 173.3 ppm corresponding to carbonyl carbon of the citric acid at the 2 and 1,3 positions, respectively. In the polyester spectra, a new broader signal emerges at ~171 ppm consistent with ester bond formation. The spectrum of the neat polymer and the polymer synthesized in the presence of ZnCl2 are represented by particularly broad signals at ~173 and 176 ppm whereas much sharper signals appear in the same areas of the spectra of the polyesters formed in the presence of MgCl2 and CaCl2 suggesting the diminished structure heterogeneity in the later samples. Relative integrations of the 173 and 176 ppm signals suggest that in the neat sample, for every unreacted carboxylic acid group at the 2 position of the citric acid there are two unreacted carboxylic acids at the 1, 3 positions consistent with the molarity of the system. Remarkably, in the CaCl2, MgCl2 and the ZnCl2 samples per every unreacted carboxylic acid at the 2 position of citric acid there are 1.6, 1.5, and 1.2 unreacted carboxylic acids at the 1, 3 positions.
Carbonyl region of 13C NMR of the glycerol-citric acid polymerization products. a Unreacted monomers; b neat polymerization; polymerization in the presence of 0.25 molar equivalent of c CaCl2, d MgCl2, e ZnCl2. To assure quantitative results the spectra were collected employing 30 % inversion pulse, 1H decoupling during acquisition only and 45 s recycle delay
Mass Spectrometry
For the mass spectrometric analysis we expanded the inventory of the chloride salts as co-reagents of the polyesterification. NMR analysis of polymers in the presence of dissolved CoCl2, CuCl2 and NiCl2 was not possible due to the paramagnetic nature of these elements. Figure 3 shows the mass spectra of the neat polymer (a) and polymer synthesized in the presence of 0.25 equivalent of CaCl2 relative to citric acid. The spectrum of the neat polymer is dominated by glycerol-rich species, namely oligomers containing two glycerols and one citric acid (G2C), four glycerols and 2 citric acids (G4C2), as well as G3C, G5C3, G6C3. The largest detectable oligomer in the spectrum has a molecular mass of 1306 and is assigned to G8C4. Many of the same species are found in the CaCl2 polymer sample with the exception of the higher molecular weight species. Notably, the abundance of species consisting of equimolar ratios of glycerol and citric acid is amplified. This result is maintained in all the polymer systems containing the divalent chloride salts. Table 1 summarizes the abundances (relative to the abundance of the G2C species) of the common oligomers. The relative abundance of the GC, G2C2 and G3C3 species is increased an order of magnitude in the polymers synthesized in the presence of CaCl2, CoCl2, CuCl2, MgCl2 and NiCl2; the effect is less pronounced in the case of ZnCl2. Neither of the mass spectra contains a signal corresponding to dicitrate implying that the hydroxyl group of the citric acid does not participate in the esterification reactions.
High resolution mass spectra of glycerol-citric acid oligomers synthesized in neat and in the present of 0.25 eqivalent of CaCl2. All labeled species correspond to [M−H]− ions. Molecular formulas based on accurate mass measurements are consistent with oligomers containing glycerol (G) and citric acid (C) moieties
Size Exclusion Chromatography (SEC)-MS
SEC is a separation method based on the hydrodynamic volume of an analyte. Compound separation is achieved by the difference in the interactions of the eluting particles within pores of the stationary phase rather than surface interactions in traditional chromatography. In principle, large molecules, with large hydrodyamic volumes elute quickly, whereas smaller molecules interact more with the stationary phase and elute later. In the case of linear polymers, the hydrodynamic volume is directly correlated to the molecular weight; thus, the molar masses of unknown linear polymers may be estimated relative to standards. Hyperbranched and dendritic polymers exhibit different relationships between molecular weight and their hydrodynamic radii as compared with their linear counterparts (Malmström et al. 1995). Moreover, the nature of the terminating groups of branched polymers is expected to have a large effect on the polymer interaction with the solvent that, in turn, determines the hydrodynamic volume. The hydrodynamic volume of carboxylic acid terminated dendrimers in aqueous solution, for example, is strongly pH-dependent and can change up to 50 % by merely changing the pH of the solution (Newkome et al. 1993). The SEC separation in this case is therefore largely based on a complex relationship between the oligomer chemistry and architecture rather than the molar mass. As described below, species with lower molecular mass in some cases elute before the larger species, oligomer isomers appear at separate retention times.
Figure 4 illustrates the result of the SEC-MS analysis of the glycerol : citric acid 2:1 polymers prepared neat and in the presence of 0.25 equivalent of divalent cation chlorides. The depicted chromatograms are detected by the intensity of UV absorbance at 210 nm. The neat polymer features three distinctive peaks suggesting 3 distributions of products based on their hydrodynamic volumes. The peak at 5.3 min consists of a collection of oligomers, from a GC dimer to G8C4 dodecamer, and is similar in all the samples. The 6.1 min peak of the neat peak polymer is dominated by the G2C and G4C2 species. Finally, the signal at 6.7 min contains mostly the G3C2 and G2C2 species. In the case of the polymers formed in the presence of dissolved CaCl2, CoCl2, CuCl2, MgCl2 and NiCl2, the corresponding peak is shifted towards the lower retention times, split into 2 maxima and dominated by the unreacted citric acid and GC dimer. In the case of ZnCl2 the corresponding area lacks significant amounts of citric acid, features GC, G2C and G4C2 oligomers. The longer retention time of the chromatograms of the polymers formed in the presence of divalent cations is characterized by a broad distribution of products, the peaks have similar shapes and composition as determined by the MS but slightly different retention times suggesting the presence of oligomer-cation complexes. The ~6.3 min maximum is dominated by the G4C2 species whereas the ~7.3 min peak contains a number of oligomers consisting of 1:1 ratio of glycerol and citric acid. The spiking intensities of the ~7.3 min peaks in the UV chromatograms are possibly attributable to the increased cation-oligoester complex absorbance.
SEC-MS analysis of glycerol-citric acid oligomers synthesized in neat and in the presence of 0.25 eqivalent of CaCl2, CoCl2, CuCl2, MgCl2, NiCl2 and ZnCl2. All labeled species in the mass spectra correspond to [M−H]− ions. Masses are consistent with oligomers containing glycerol (G) and citric acid (C) moieties detected as is or in clusters with formic acid (FA) contained in the eluent
Discussion
Our results show that it is possible to synthesize polyesters of appreciable length from unactivated monomers in the dry state under mild heat. The esterification reaction may be acid catalyzed as it proceeds at pH = 2 when naturally buffered by the citric acid and free acid groups of the formed citrate esters. We have previously shown that malic acid polymerizes under similar thermal conditions (Mamajanov et al. 2014). The malic acid oligomer in their study predominantly consisted of dimers and trimers. Our systems produced significant amounts of tetramer, pentamer and hexamer species as evidenced in the MS and SEC-MS analyses. The polymerization in both systems occurs in the dehydrated state that, in the case of malic acid, is described by a diffusionally limited amorphous solid or crystalline state, whereas in the case of glycerol and citric acid is represented by a clear solution or a eutectic mixture, which allows for a higher diffusional mobility and for this reason higher reaction rate and degree of polymerization.
The excellent chelation ability of citric acid is well documented (Changa 1983; Hedwig et al. 1980; Müller et al. 1997; Singh et al. 1991; Zelenin 2007; Zhou et al. 2005), Citric acid is therefore widely used as a water softening agent and in a variety of cleaning and decalcification solutions. Structural studies of the complexes suggest the involvement of the two carboxylic groups at the 1 and 2 positions of citric acid as well as the hydroxyl group (Zhou et al. 2005). It is plausible that complexes of this kind are formed in our samples, possibly in the wet phase prior to drying. The complex formation deactivates the carboxylic acids at the 2 and the 1 positions of citric acid to esterification, leaving the 3 position free to react. This explains the higher relative incorporation of 3 (equivalent to 1) position carboxylic acids into the polyester as evidenced by the 13C NMR spectrum (Fig. 2). In general, the addition of the divalent cation chlorides to the glycerol-citric acid polymerization systems favors a higher degree of citric acid incorporation as indicated by the MS and SEC-MS data. The high ratio of citric acid to glycerol in the polymer ensures a large number of free carboxylic acid sidechains are available and capable of metal binding. Citric acid deactivation by complexation, however, results in lower conversion and decreased overall degree of polymerization with the notable exception of the ZnCl2 system. The polymer synthesized in the presence of 0.125 and 0.25 equivalents of ZnCl2 relative to citric acid had no significant impact on conversion or the length of the product, whereas 0.5 equivalent systems produced insoluble products suggesting higher degree of polymerization, crosslinking or formation of insoluble cation-polymer complexes. ZnCl2 is commonly used as a moderate-strength Lewis acid catalyst in the Fisher indole synthesis and Friedel-Crafts acylations, as well as in dehydration reactions (Kuchkarev and Shuikin 1956). Both properties of ZnCl2 may catalyze esterification. The products of the ZnCl2 system are biased towards citric acid-rich species but to a lesser degree compared to the rest of the divalent cation chloride systems suggesting the catalytic processes compete with citric acid complexation in this case.
Many proteins are capable of tight ligand binding. This ability facilitates a number of essential functions, such as enzymatic and receptor activities, self-organization into multicomponent complexes and ligand transport. Receptor activity and multicomponent protein complexes most likely emerged at the later evolutionary stage, whereas catalytic agents were likely necessary to govern the network prebiotic reactions during early stages of chemical evolution. While the prebiotic synthesis of functional peptides has proven challenging, branched polyesters may provide an alternative. As depicted in the chromatograms in the Fig. 4 and S3, divalent cations affect the retention times of certain species suggesting the presence of oligomers capable of cation binding. The in-depth study of cation binding and catalytic properties of these structures is underway in our lab.
The emerging field of so-called smart polymers involves studies of materials that can change in response to external stimuli (Chopra 2002). Such materials can be sensitive to factors, such as pH, temperature, humidity, light intensity, or an electrical or magnetic field and can respond by undergoing conformational change, altering their electronic properties effecting their color and/or conductivity. Smart materials possess emergent properties and are, therefore, conceptually of interest to the problem of the origin of life. The branched polyesters described here are an example of a rudimentary prebiotically plausible smart material. The citric acid-glycerol polymerization system yields a heterogeneous mixture of oligomers of various lengths, structures and sequences. As evidenced by the presented results, the length and the sequence of these oligomers can be controlled by introducing stimuli in the form of divalent cations at the polyesterification step. Previously described polyesterification under far from equilibrium conditions achieved by heat and level of hydration (day/night) cycling (Mamajanov et al. 2014) provides an attractive platform for further investigation of the emergent properties of citric acid-glycerol oligomers. Under these conditions the condensation occurs during the dry day phase while a certain amount of hydrolysis takes place during the subsequent hydrated night phase. Repeated cycles assure accumulation of more stable oligomer structures, which are potentially stabilized by cations, and recycling of the remaining structures. We are currently pursuing this investigation.
Conclusions
We have demonstrated the synthesis of branched polyesters at moderate temperature and in the dry state from unactivated monomers without the use of unnaturally occurring catalysts. The degree of polymerization of equimolar glycerol-citric acid is high enough to cause gelation, whereas the polymer formed from an excess of glycerol contains up to dodecamer species. The resulting polymer is heterogeneous with the respect to length and likely with respect to isomerism. Introduction of divalent cation chlorides during polymerzation favors low molecular weight oligomers and a higher degree of citric acid incorporation. This feature of the glycerol-citric acid system formulates an attractive avenue of research into smart materials.
References
Astruc D, Chardac F (2001) Dendritic catalysts and dendrimers in catalysis. Chem Rev 101:2991–3024. doi:10.1021/cr010323t
Berg JM, Tymoczko JL, Stryer L (2012) Biochemistry, 7th edn. W.H. Freeman, New York
Bernstein MP, Dworkin JP, Sandford SA, Cooper GW, Allamandola LJ (2002) Racemic amino acids from the ultraviolet photolysis of interstellar ice analogues. Nature 416:401–403. doi:10.1038/416401a
Bhyrappa P, Young JK, Moore JS, Suslick KS (1996) Dendrimer-metalloporphyrins: synthesis and catalysis. J Am Chem Soc 118:5708–5711. doi:10.1021/ja953474k
Breslow R (1959) On the mechanism of the formose reaction. Tetrahedron Lett 1:22–26. doi:10.1016/S0040-4039(01)99487-0
Butch C, Cope ED, Pollet P, Gelbaum L, Krishnamurthy R, Liotta CL (2013) Production of tartrates by cyanide-mediated dimerization of glyoxylate: a potential abiotic pathway to the citric acid cycle. J Am Chem Soc 135:13440–13445. doi:10.1021/ja405103r
Changa DM (1983) The binding of free calcium ions in aqueous solution using chelating agents, phosphates and poly(acrylic acid). J Am Oil Chem Soc 60:618–622. doi:10.1007/BF02679800
Chen S, Zhang X-Z, Cheng S-X, Zhuo R-X, Gu Z-W (2008) Functionalized amphiphilic hyperbranched polymers for targeted drug delivery. Biomacromolecules 9:2578–2585. doi:10.1021/bm800371n
Chopra I (2002) Review of state of art of smart structures and integrated systems. AIAA J 40:2145–2187. doi:10.2514/2.1561
Cody GD, Boctor NZ, Hazen RM, Brandes JA, Morowitz HJ, Yoder HS Jr (2001) Geochemical roots of autotrophic carbon fixation: hydrothermal experiments in the system citric acid, H2O-(±FeS)−(±NiS). Geochim Cosmochim Acta 65:3557–3576. doi:10.1016/S0016-7037(01)00674-3
Cooper G, Kimmich N, Belisle W, Sarinana J, Brabham K, Garrel L (2001) Carbonaceous meteorites as a source of sugar-related organic compounds for the early Earth. Nature 414:879–883
Cooper G, Reed C, Nguyen D, Carter M, Wang Y (2011) Detection and formation scenario of citric acid, pyruvic acid, and other possible metabolism precursors in carbonaceous meteorites. Proc Natl Acad Sci 108:14015–14020. doi:10.1073/pnas.1105715108
Fahnestock S, Rich A (1971) Ribosome-catalyzed polyester formation. Science 173(3994):340–343. doi:10.1126/science.173.3994.340
Fahnestock S, Neumann H, Shashoua V, Rich A (1970) Ribosome-catalyzed ester formation. Biochemistry 9:2477–2483. doi:10.1021/bi00814a013
Ferris JP, Hill AR, Liu R, Orgel LE (1996) Synthesis of long prebiotic oligomers on mineral surfaces. Nature 381:59–61. doi:10.1038/381059a0
Flory PJ (1941a) Molecular size distribution in three dimensional polymers. I. Gelation1. J Am Chem Soc 63:3083–3090. doi:10.1021/ja01856a061
Flory PJ (1941b) Molecular size distribution in three dimensional polymers. II. Trifunctional branching units. J Am Chem Soc 63:3091–3096. doi:10.1021/ja01856a062
Flory PJ (1941c) Molecular size distribution in three dimensional polymers. III. Tetrafunctional branching units. J Am Chem Soc 63:3096–3100. doi:10.1021/ja01856a063
Flory PJ (1952) Molecular size distribution in three dimensional polymers. VI. Branched polymers containing A—R—Bf-1 type units. J Am Chem Soc 74:2718–2723. doi:10.1021/ja01131a008
Fox SW, Harada K (1958) Thermal copolymerization of amino acids to a product resembling protein. Science 128:1214
Fréchet JMJ, Hawker CJ (1995) Hyperbranched polyphenylene and hyperbranched polyesters: new soluble, three-dimensional, reactive polymers. React Funct Polym 26:127–136. doi:10.1016/1381-5148(95)00010-D, Proceedings of the 6th International Conference on Polymer Supported Reactions in Organic Chemistry
Gao C, Yan D (2004) Hyperbranched polymers: from synthesis to applications. Prog Polym Sci 29:183–275. doi:10.1016/j.progpolymsci.2003.12.002
Gao C, Xu Y, Yan D, Chen W (2003) Water-soluble degradable hyperbranched polyesters: novel candidates for drug delivery? Biomacromolecules 4:704–712. doi:10.1021/bm025738i
Halpern JM, Urbanski R, Weinstock AK, Iwig DF, Mathers RT, von Recum HA (2014) A biodegradable thermoset polymer made by esterification of citric acid and glycerol. J Biomed Mater Res A 102:1467–1477. doi:10.1002/jbm.a.34821
Hedwig G, Liddle J, Reeves R (1980) Complex formation of nickel (II) ions with citric acid in aqueous solution: a potentiometric and spectroscopic study. Aust J Chem 33:1685–1693
Hollis JM, Lovas FJ, Jewell PR, Coudert LH (2002) Interstellar antifreeze: ethylene glycol. Astrophys J 571:L59. doi:10.1086/341148
Hu M, Chen M, Li G, Pang Y, Wang D, Wu J, Qiu F, Zhu X, Sun J (2012) Biodegradable hyperbranched polyglycerol with ester linkages for drug delivery. Biomacromolecules 13:3552–3561. doi:10.1021/bm300966d
Kienle RH, van der Meulen PA, Petke FE (1939) The polyhydric alcohol-polybasic acid reaction. III. Further studies of the glycerol-phthalic anhydride reaction. J Am Chem Soc 61:2258–2268. doi:10.1021/ja01878a001
Kim YH, Webster OW (1990) Water soluble hyperbranched polyphenylene: “a unimolecular micelle?”. J Am Chem Soc 112:4592–4593. doi:10.1021/ja00167a094
Kirkorian K, Ellis A, Twyman LJ (2012) Catalytic hyperbranched polymers as enzyme mimics; exploiting the principles of encapsulation and supramolecular chemistry. Chem Soc Rev 41:6138–6159. doi:10.1039/c2cs35238a
Kolhe P, Misra E, Kannan RM, Kannan S, Lieh-Lai M (2003) Drug complexation, in vitro release and cellular entry of dendrimers and hyperbranched polymers. Int J Pharm 259:143–160. doi:10.1016/S0378-5173(03)00225-4
Kuchkarev AB, Shuikin NI (1956) Mechanism of hydration and dehydration reactions in presence of zinc halides. Bull Acad Sci USSR Div Chem Sci 5:1511–1519. doi:10.1007/BF01177533
Lahav N, White D, Chang S (1978) Peptide formation in the prebiotic era: thermal condensation of glycine in fluctuating clay environments. Science 201(4350):67–69. doi:10.1126/science.663639
Liu L, Breslow R (2003) Dendrimeric pyridoxamine enzyme mimics. J Am Chem Soc 125:12110–12111. doi:10.1021/ja0374473
Liu R, Orgel LE (1997) Efficient oligomerization of negatively-charged β-amino acids at −20 °C. J Am Chem Soc 119:4791–4792. doi:10.1021/ja9702529
Malmström E, Hult A (1996) Kinetics of formation of hyperbranched polyesters based on 2,2-bis(methylol)propionic acid. Macromolecules 29:1222–1228. doi:10.1021/ma951084c
Malmström E, Johansson M, Hult A (1995) Hyperbranched aliphatic polyesters. Macromolecules 28:1698–1703. doi:10.1021/ma00109a049
Mamajanov I, MacDonald PJ, Ying J, Duncanson DM, Dowdy GR, Walker CA, Engelhart AE, Fernández FM, Grover MA, Hud NV, Schork FJ (2014) Ester formation and hydrolysis during wet–dry cycles: generation of far-from-equilibrium polymers in a model prebiotic reaction. Macromolecules 47:1334–1343. doi:10.1021/ma402256d
Martin RB (1998) Free energies and equilibria of peptide bond hydrolysis and formation. Biopolymers 45:351–353. doi:10.1002/(SICI)1097-0282(19980415)45:5<351::AID-BIP3>3.0.CO;2-K
Miller SL, Urey HC (1959) Organic Compound Synthes on the Primitive Eart Several questions about the origin of life have been answered, but much remains to be studied. Science 130(3370):245–251. doi:10.1126/science.130.3370.245
Morowitz HJ, Kostelnik JD, Yang J, Cody GD (2000) The origin of intermediary metabolism. Proc Natl Acad Sci 97:7704–7708. doi:10.1073/pnas.110153997
Müller B, Kläger W, Kubitzki G (1997) Metal chelates of citric acid as corrosion inhibitors for zinc pigment. Corros Sci 39:1481–1485. doi:10.1016/S0010-938X(97)00061-9
Newkome G, Young J, Baker G (1993) Cascade polymers. 35. pH dependence of hydrodynamic radii of acid-terminated dendrimers. Macromolecules 26:2394–2396. doi:10.1021/ma00061a041
Orgel LE (2003) Some consequences of the RNA world hypothesis. Orig Life Evol Biosph 33:211–218. doi:10.1023/A:1024616317965
Peltzer ET, Bada JL (1978) α-hydroxycarboxylic acids in the Murchison meteorite. Nature 272:443–444. doi:10.1038/272443a0
Pizzarello S, Wang Y, Chaban GM (2010) A comparative study of the hydroxy acids from the Murchison, GRA 95229 and LAP 02342 meteorites. Geochim Cosmochim Acta 74:6206–6217. doi:10.1016/j.gca.2010.08.013
Prabaharan M, Grailer JJ, Pilla S, Steeber DA, Gong S (2009) Folate-conjugated amphiphilic hyperbranched block copolymers based on Boltorn® H40, poly(l-lactide) and poly(ethylene glycol) for tumor-targeted drug delivery. Biomaterials 30:3009–3019. doi:10.1016/j.biomaterials.2009.02.011
Ricardo A, Frye F, Carrigan MA, Tipton JD, Powell DH, Benner SA (2006) 2-hydroxymethylboronate as a reagent to detect carbohydrates: application to the analysis of the formose reaction. J Org Chem 71:9503–9505. doi:10.1021/jo061770h
Sagi VN, Punna V, Hu F, Meher G, Krishnamurthy R (2012) Exploratory experiments on the chemistry of the “glyoxylate scenario”: formation of ketosugars from dihydroxyfumarate. J Am Chem Soc 134:3577–3589. doi:10.1021/ja211383c
Schneider-Bernloehr H, Lohrmann R, Sulston J, Weimann BJ, Orgel LE, Miles HT (1968) Non-enzymic synthesis of deoxyadenylate oligonucleotides on a polyuridylate template. J Mol Biol 37:151–155. doi:10.1016/0022-2836(68)90079-X
Schwartz A, Fox S (1964) Thermal synthesis of internucleotide phosphodiester linkages. Biochim Biophys Acta 87:694. doi:10.1016/0926-6550(64)90290-7
Schwendinger MG, Tattler R, Saetia S, Liedl KR, Kroemer RT, Rode BM (1995) Salt induced peptide formation: on the selectivity of the copper induced peptide formation under possible prebiotic conditions. Inorg Chim Acta 228:207–214. doi:10.1016/0020-1693(94)04186-Y
Singh RP, Yeboah YD, Pambid ER, Debayle P (1991) Stability constant of the calcium-citrate(3-) ion pair complex. J Chem Eng Data 36:52–54. doi:10.1021/je00001a015
Tasaka F, Ohya Y, Ouchi T (2001) One-pot synthesis of novel branched polylactide through the copolymerization of lactide with mevalonolactone. Macromol Rapid Commun 22:820–824. doi:10.1002/1521-3927(20010701)22:11<820::AID-MARC820>3.0.CO;2-7
Weber AL (1989) Thermal synthesis and hydrolysis of polyglyceric acid. Orig Life Evol Biosph 19:7–19. doi:10.1007/BF01808284
Weber AL, Orgel LE (1978) Amino acid activation with adenosine 5′-phosphorimidazolide. J Mol Evol 11:9–16. doi:10.1007/BF01768020
Weimann BJ, Lohrmann R, Orgel LE, Schneider-Bernloehr H, Sulston JE (1968) Template-directed synthesis with adenosine-5′-phosphorimidazolide. Science 161(3839):387. doi:10.1126/science.161.3839.387
Williams RJ, Gabriel A, Andrews RC (1928) The relation between the hydrolysis equilibrium constant of esters and the strengths of the corresponding acids. J Am Chem Soc 50:1267–1271. doi:10.1021/ja01392a005
Wooley KL, Hawker CJ, Lee R, Fréchet JMJ (1994) One-step synthesis of hyperbranched polyesters. Molecular weight control and chain end functionalization. Polym J 26:187–197. doi:10.1295/polymj.26.187
Zelenin OY (2007) Interaction of the Ni2+ ion with citric acid in an aqueous solution. Russ J Coord Chem 33:346–350. doi:10.1134/S1070328407050065
Zhou Z-H, Deng Y-F, Wan H-L (2005) Structural diversities of Cobalt(II) coordination polymers with citric acid. Cryst Growth Des 5:1109–1117. doi:10.1021/cg0496282
Acknowledgments
We thank Dr. Leslie Gelbaum for his help in the design of NMR experiments and Prof. Nicholas Hud for helpful discussions. This work is supported by Simons Foundation Collaboration on the Origin of Life Fellowship SCOL 292864 (I.M.) and Investigator Award SCOL 302497 (M.P.C., J.P.D.), as well as the NASA Astrobiology Institute award to the Carnegie Institution for Science (I.M., G.D.C.) and The Goddard Center for Astrobiology (M.P.C., J.P.D.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Paper presented at ORIGINS 2014, Nara Japan, July 6–11 2014.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
1H NMR spectra of a) glycerol triacetate; b) glycerol diacetate, mixture of isomers, contains mono- and triacetate c) glycerol monoacetate, mixture of isomers, contains di- and triacetate. The samples were buffered by citric acid. (GIF 42 kb)
High Resolution Image
(TIFF 37492 kb)
Figure S2
ACD chemical shift prediction for a characteristic glycerol-citric acid polymer fragment. Predicted a) 1H and b) 13C chemical shifts. (GIF 51 kb)
High Resolution Image
(TIFF 18169 kb)
Figure S3
SEC analysis (UV trace) of glycerol-citric acid oligomers synthesized in neat and in the presence of 0.125, 0.25 and 0.5 eqivalents of MgCl2. Similar behavior is observed in the CaCl2, CoCl2, CuCl2, NiCl2 and ZnCl2 systems. (GIF 71 kb)
High Resolution Image
(TIFF 14409 kb)
Rights and permissions
About this article
Cite this article
Mamajanov, I., Callahan, M.P., Dworkin, J.P. et al. Prebiotic Alternatives to Proteins: Structure and Function of Hyperbranched Polyesters. Orig Life Evol Biosph 45, 123–137 (2015). https://doi.org/10.1007/s11084-015-9430-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-015-9430-9