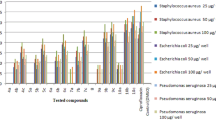
Four new 1,3,4-bis-oxadiazole derivatives were synthesized, two of them bearing quaternary ammonium salts. The newly synthesized bis-1,3,4-oxadiazoles were investigated for their antibacterial activity against various Gram-positive and Gram-negative strains of bacteria. The target products were prepared from 2-(dimethylamino)ethyl methacrylate (DMAEMA) or 2-(diethylamino)ethyl methacrylate (DEAEMA) via reactions with hexanedioic acid. The structures of all synthesized compounds were confirmed by IR, 1H-NMR, and 13C-NMR spectroscopy. The results of biological activity testing showed that two compounds exhibited the best antibacterial activity against Citrobacterfreundii (ATCC 8090) and Methicillin-resistant Staphylococcus aureus (ATCC 43300) strains.

Similar content being viewed by others
References
A. Pace and P. Pierro, Org. Biomol. Chem., 7, 4337 – 4348 (2009).
K. Bethge, H. H. Pertz, and K. Rehse, Arch. Pharm., 338, 78 – 86 (2005).
D. K. Dalvie, A. S. Kalgutkar, S. C. Khojasteh-Bakht, et al., Chem. Res. Toxicol., 15, 269 – 299 (2002).
E. A. Ryzhova, A. G. Koryakova, E. A. Bulanova, et al., Pharm. Chem. J., 43, 148 – 153 (2009).
S. Balachandran, A. Rodge, P. K. Gadekar, et al., Bioorg. Med. Chem. Lett. , 19, 4773 – 4776 (2009).
J. Boström A. Hogner, A. Llinás, et al., J. Med. Chem., 55, 1817 – 1830 (2012).
A. C. L. Leite, R. F. Vieira, A. R. de Faria, et al., Il Farmaco, 55, 719 – 724(2000).
J. V. dos Anjos, D. Sinou, S. J. de Melo, and R. M. Srivastava, Carbohydr. Res., 342, 2440 – 2449 (2007).
J. Ziga, Current Org. Synth., 12, 1 – 6 (2015).
G. A. Allan, J. I. Gedge, A. N. Nedderman, et al., Xenobiotica , 36, 399 – 418 (2006).
J. G. Allen, M. J. Blackburn, and S. M. Caldwell, Xenobiotica , 1, 3 – 12 (1971).
J. Saunders, M. Cassidy, S. B. Freedman, et al., J. Med. Chem. , 33, 1128 – 1138 (1990).
K. P. Bateman, L. Trimble, N. Chauret, et al., J. Mass Spectrom., 41, 771 – 780 (2006).
A. Hall, S. H. Brown, A. Chowdhury, et al., Bioorg. Med. Chem. Lett. , 17, 4450 – 4455 (2007).
Y. Ducharme, M. Blouin, C. Brideau, et al., ACS Med. Chem. Lett., 1, 170 – 174 (2010).
F. Huguet, A. Melet, R. Alves de Sousa, et al., Chem. Med. Chem., 7 1020 – 1030 (2012).
R. S. Ferreira, C. Bryant, K. K. H. Ang, et al., J. Med. Chem., 52, 5005 – 5008 (2009).
F. Z. Dörwald: Optimization for Medicinal Chemists: Pharmacokinetic Properties of Functional Groups and Organic Compounds, John Wiley & Sons: New York (2013), pp. 118 – 119.
Q. Xiaoshuai, L. Yancai, Z. Fang, et al., Appl. Surf. Sci., 328, 183 – 192 (2015).
G. Sauvet, S. Dupond, K. Kazmierski, and J. Chojnowski, J. Appl. Polym. Sci.. 75, 1005–1012 (2000).
G. A. Domagk, Deut. Med. Wochenschr., 61, 829 – 832 (1935).
G. L. Jeffrey, N. C. Peter, A. F. Preston, and H. W. James, React. Funct. Polym., 77, 39 – 46 (2014).
(a) Z. Anqiang, L. Qiongqiong, L. Yufeng, et al., React. Funct. Polym., 88, 39 – 46(2015); (b) W. Chamari, B. Marianna, B. Lívia, et al., Clin. Pharm., 4, 73 – 75 (2013).
T. Ulas and G. P. Spyros, Curr. Opin. Biotech., 33, 296 – 304 (2015).
N. Basillico, M. Migotto, D. P. Ilboudo, et al., Bioorg. Med. Chem., 23, 4681 – 4687 (2015).
L. Yaling, L. Qiongqiong; C. Liujun, et al., React. Funct. Polym., 85, 36 – 44 (2014).
Z. S. Xu, W. Ning, C. Di, et al., Chin. Chem. Lett., 22, 887 – 890 (2011).
R. B. Yashumati, P. Ashutosh, J. Vivek, and K. Dharma, Saudi Pharm. J., 22, 290 – 302 (2014).
H. Guo-Qiang, H. Li-Li, W. Guo-Qiang, et al., Acta Pharm. Sinica , 47, 1017 – 1022 (2012).
B. S. Furniss, A. J. Hannford, P. W. G. Smith, and A. R. Tatchell, in: Vogel’s Text Book of Practical Organic Chemistry. 5th Edition, John Wiley & Sons: New York (1989), p. 1076.
A. K. Behera, R. K. Behera, R. Pradhan, et al., Indian J. Heterocycl. Chem., 16, 167 – 170 (2006).
L. Guiqian, W. Dingcai, F. Ruowen, React. Funct. Polym., 67, 355 – 366 (2007).
M27-A2 Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved Standard, 2nd Edition, Clinical and Laboratory Standards Institute (United States), NCCLS Document, Vol. 22, No. 5 (2002).
S. O. Podunavac-Kuzmanovic, D. D. Cvetkovic, and D. J. Barna, J. Serb. Chem. Soc., 73 , 967 – 978 (2008).
Acknowledgements
Prof. Dr. Taoufik Rohand thanks the University Cadi Ayyad and specially the Faculty Polidisciplinaire of Safi for the profesorship and the Laboratory of Analytical and Molecular Chemistry for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rohand, T., Ramli, Y., Baruah, M. et al. Synthesis, Structure Elucidation and Antimicrobial Properties of New Bis-1,3,4-Oxadiazole Derivatives. Pharm Chem J 53, 150–154 (2019). https://doi.org/10.1007/s11094-019-01969-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-019-01969-2