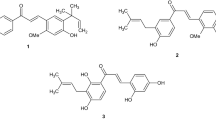
Newly designed chalconeimines were synthesized, characterized and evaluated for their in vitro antioxidant and antibacterial effectiveness. Results of antioxidant activity assay reveal that all the tested compounds possess good to moderate antioxidant activity which is lower in comparison to that of a standard drug (gallic acid). On the other hand, all the synthesized compounds were found to exhibit a considerably wider spectrum of antibacterial activity, but it was also narrower in comparison to that of a standard drug (ciprofloxacin). Elucidation of structure—activity relationships revealed that electron donating groups (-OH, -OCH3) contribute more to antioxidant potency, whereas electron withdrawing groups (-Cl) impart better antibacterial effectiveness. Moreover, results of drug-likeness studies indicate that a reasonable correlation exists between the drug-like properties and antioxidant activity of the synthesized chalconeimines.


Similar content being viewed by others
References
R. Pingaew, A. Saekee, P. Mandi, et al., Eur. J. Med. Chem., 85, 65 – 76 (2014).
S. Maayan, N. Ohad, K. Soliman, et al., Bioorg. Med. Chem.,13(2), 433 – 441 (2005).
K. Maria, H. Dimitra, and G. Maria, Med. Chem.,4, 586 – 596 (2008).
S. Sinha, B. Medhi, and R. Sehgal, J. Mod. Med. Chem.,1, 64 – 77 (2013).
A. Detsi, M. Majdalani, C. A. Kontogiorgis, and D. Hadjipavlou-Litina, Bioorg. Med. Chem.,20, 8073–8085 (2009).
B. Ngameni, V. Kuete, P. Ambassa, et al., Med. Chem.,3(3), 233 – 237 (2013).
H. Zhang, J. Liu, J. Sun, et al., Bioorg. Med. Chem.,20 3212 – 3218 (2012).
H. Iqbal, V. Prabhakar, A. Sangith, and B. Chandrika, Med. Chem. Res.,23, 4383 – 4394 (2014).
M. Rudrapal, R. Siva Satyanandam, T. Sri Swaroopini, et al., Med. Chem. Res.,22 (6), 2840 – 2846 (2013).
C. M. da Silva, D. L. da Silva, L. V. Modolo, et al., J. Adv. Res.,2, 1 – 8 (2011).
M. Rudrapal and B. De, Int. Res. J. Pure App. Chem.,3(3), 232 – 249 (2013).
A. A. Al-Amiery, A. D. H. Kadhum, M. Shamel, et al., Med. Chem. Res.,23, 236 – 242 (2014).
N. A. Ghanwate, A. W. Raut, and A. G. Doshi, Oriental J. Chem.,24(2), 733 – 736 (2008).
S. G. Patil, P. S. Utale, S. B. Gholse, et al., J. Chem. Pharm. Res., 4(1), 501 – 507 (2012).
S. Patil, P. Utale, S. Gholse, et al., Asian J. Biochem. Pharm. Res.,3(2), 114 – 122 (2013).
S. Das, I. Mitra, S. Batuta, et al., Bioorg. Med. Chem.,24, 5050 – 5054 (2014).
M. Liang, X. Yu, L. Cong, et al., Bioorg. Med. Chem.,21, 6763–6770 (2013).
M. S. Brewer, Compr. Rev. Food Sci. Food Safety,10, 221 – 242 (2011).
B. Uttara, A. V. Singh, P. Zamboni, and R. T. Mahajan, Curr. Neuropharmacol.,7, 65 – 74 (2009).
E. A. Shalaby and M. M. Sanaa Shanab, Afr. J. Pharm. Pharmacol.,7(10), 528 – 539 (2013).
M. Rudrapal, T. Mariya Babu, R. Ravi Chandra, et al., Anti-Infect. Agents,12 (2), 198 – 205 (2014).
E. H. Kerns and L. Di, Drug-like Properties: Concepts, Structure Design and Methods, Academic Press, New York (2008), pp. 7 – 9.
B. S. Furniss, A. J. Hannaford, P. W. G. Smith, and A. R. Tatchell, Vogel′xs Text Book of Practical Organic Chemistry, John Willey & Sons, Inc., New York (1989), pp. 256.
S. R. Pattan, M. S. Ali, J. S. Pattan, et al., Indian J. Chem.45B, 1929 – 1932 (2006).
J. G. Collee, J. P. Duguid, M. G. Fraser, B. P. Marmion and M. McCartney, Practical Medical Microbiology, Churchill Livingstone, London (1989), pp. 346.
R. M. Silverstein and F. X. Webster, Spectrometric Identification of Organic Compounds, John Wiley and Sons, Inc., New York (1963), pp. 203, 213.
A. Crozier, I. B. Jaganath, and M. N. Clifford, Nat. Prod. Rep.,26, 1001 – 1043 (2009).
R. Tsao, Nutrients, 2, 1231 – 1246 (2010).
A. Kunwar and K. I. Priyadarshini, J. Med. Allied Sci.,1(2), 53 – 60 (2011).
B. Kim, O. Kwang-Joong, J. Chun, and K. Hwang, Bull. Korean Chem. Soc.29(6), 1125 – 1130 (2008).
K. Parikh and D. Joshi, Med. Chem. Res.,22, 3688 – 3697 (2013).
C. A. Lipinski, F. Lombardo, B. W. Dominy, and P. J. Feeney, Adv. Drug Deliv. Rev.,23, 3 – 26 (1997).
Acknowledgements
The authors express their sincere thanks to Dr. K. Ravishankar, Principal, Sri Sai Aditya Institute of Pharmaceutical Sciences and Research, Surampalem, E. G. Dist., Andhra Pradesh (India) for his generous help and co-operation in studying out the antioxidant activity of synthesized compounds. The authors also wish to thank Mr. N. Sathish Reddy, Vice-Chairman, Aditya Educational Institutions, Surampalem, E. G. Dist., Andhra Pradesh (India) for providing necessary laboratory facilities to carry out the work.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rudrapal, M., Sowmya, M.P.K. Design, Synthesis, Drug-Likeness Studies and Bio-Evaluation of Some New Chalconeimines. Pharm Chem J 53, 814–821 (2019). https://doi.org/10.1007/s11094-019-02084-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-019-02084-y