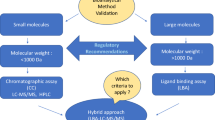
The development and validation of ligand binding assays used in the support of pharmacokinetic studies has been the focus of various workshops and publications in recent years, all in an effort to establish a guidance document for standardization of these bioanalytical methods. This summary report of the workshop from 2003 focuses on the issues discussed in presentations and notes points of discussion and areas of consensus among the participants.
Similar content being viewed by others
References
K. J. Miller R. R. Bowsher A. Celniker J. Gibbons S. Gupta J. W. Lee J. S. J. Swanson W. C. Smith R. S. Weiner (2001) ArticleTitleWorkshop on bioanalytical methods validation for macromolecules: summary report Pharm. Res. 18 1373–1383 Occurrence Handle10.1023/A:1013062600566 Occurrence Handle11683255
B. DeSilva W. Smith R. Weiner M. Kelley J. Smolec B. Lee M. Khan R. Tacey H. Hill A. Celniker (2003) ArticleTitleRecommendations for the bioanalytical method validation of ligand-binding assays tosupport pharmacokinetic assessments of macromolecules Pharm. Res. 20 IssueID11 1885–1900 Occurrence Handle10.1023/B:PHAM.0000003390.51761.3d Occurrence Handle14661937
International Conference on Harmonization. ICH Q2A. Text on Validation of Analytical Procedures. Federal Register. 1995;60 FR 11260. http://www.fda.gov/cder/guidance/ichq2a.pdf..
International Conference on Harmonization. ICH Q2B. Validation of Analytical Procedures Methodology. Federal Register. 1997;62 FR 27463. http://www.fda.gov/cder/guidance/1320fnl.pdf..
US Department of Health and Human Services. Draft Guidance for Industry: Analytical Procedures and Methods Validation, Chemistry, Manufacturing and Controls Documentation. Rockville, MD:US Dept of Health and Human Services, Food and Drug Administration. Aug 2000. http://www.fda.gov/cder/guidance/2396dft.pdf..
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smolec, J., DeSilva, B., Smith, W. et al. Bioanalytical Method Validation for Macromolecules in Support of Pharmacokinetic Studies. Pharm Res 22, 1425–1431 (2005). https://doi.org/10.1007/s11095-005-5917-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-005-5917-9