ABSTRACT
Purpose
To model and interpret drug distribution in the dermis and underlying tissues after topical application which is relevant to the treatment of local conditions.
Methods
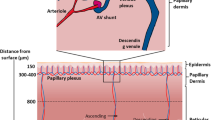
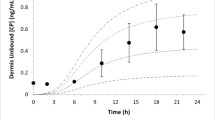
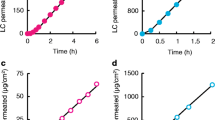
We created a new physiological pharmacokinetic model to describe the effect of blood flow, blood protein binding and dermal binding on the rate and depth of penetration of topical drugs into the underlying skin. We used this model to interpret literature in vivo human biopsy data on dermal drug concentration at various depths in the dermis after topical application of six substances. This interpretation was facilitated by our in vitro human dermal penetration studies in which dermal diffusion coefficient and binding were estimated.
Results
The model shows that dermal diffusion alone cannot explain the in vivo data, and blood and/or lymphatic transport to deep tissues must be present for almost all of the drugs tested.
Conclusion
Topical drug delivery systems for deeper tissue delivery should recognise that blood/lymphatic transport may dominate over dermal diffusion for certain compounds.





Similar content being viewed by others
REFERENCES
Schaefer H, Stuttgen G. Absolute concentrations of an antimycotic agent, econazole, in the human skin after local application. Arzneimittelforschung. 1976;26:432–5.
Schaefer H, Zesch A. Penetration of vitamin A acid into human skin. Acta Derm Venereol Suppl (Stockh). 1975;74:50–5.
Zesch A, Schaefer H. Penetration of radioactive hydrocortisone in human skin from various ointment bases. II. In vivo-experiments (author’s transl). Arch Dermatol Forsch. 1975;252:245–56.
Schaefer H, Zesch A, Stuttgen G. Penetration, permeation, and absorption of triamcinolone acetonide in normal and psoriatic skin. Arch Dermatol Res. 1977;258:241–9.
Schaefer H, Stuttgen G, Zesch A, Schalla W, Gazith J. Quantitative determination of percutaneous absorption of radiolabeled drugs in vitro and in vivo by human skin. Curr Probl Dermatol. 1978;7:80–94.
Singh P, Roberts MS. Effects of vasoconstriction on dermal pharmacokinetics and local tissue distribution of compounds. J Pharm Sci. 1994;83:783–91.
Singh P, Roberts MS. Blood-flow measurements in skin and underlying tissues by microsphere method—application to dermal pharmacokinetics of polar nonelectrolytes. J Pharm Sci. 1993;82:873–9.
Gupta E, Wientjes MG, Au JL. Penetration kinetics of 2′,3′-dideoxyinosine in dermis is described by the distributed model. Pharm Res. 1995;12:108–12.
Cross SE, Roberts MS. Defining a model to predict the distribution of topically applied growth factors and other solutes in excisional full-thickness wounds. J Invest Dermatol. 1999;112:36–41.
Kretsos K, Kasting GB, Nitsche JM. Distributed diffusion-clearance model for transient drug distribution within the skin. J Pharm Sci. 2004;93:2820.
Kretsos K, Kasting GB. A geometrical model of dermal capillary clearance. Math Biosci. 2007;208:430–53.
Kretsos K, Miller MA, Zamora-Estrada G, Kasting GB. Partitioning, diffusivity and clearance of skin permeants in mammalian dermis. Int J Pharm. 2008;346:64–79.
Dedrick RL, Flessner MF, Collins JM, Schultz JS. Is the peritoneum a membrane? ASAIO J. 1982;1–8.
Danckwerts PV. Continuous flow systems: distribution of residence times. Chem Eng Sci. 1953;2:1–13.
Roberts MS, Rowland M. A dispersion model of hepatic elimination: 1. Formulation of the model and bolus considerations. J Pharmacokinet Biopharm. 1986;14:227–60.
Anissimov YG, Roberts MS. Diffusion modeling of percutaneous absorption kinetics: 4. Effects of a slow equilibration process within stratum corneum on absorption and desorption kinetics. J Pharm Sci. 2009;98:772–81.
Anissimov YG, Roberts MS. Diffusion modeling of percutaneous absorption kinetics: 1. Effects of flow rate, receptor sampling rate and viable epidermal resistance for a constant donor concentration. J Pharm Sci. 1999;88:1201–9.
Wilke CR, Chang P. Correlation of diffusion coefficients in dilute solutions. Aiche J. 1955;1:264–70.
Cross SE, Roberts MS. Dermal blood flow, lymphatics, and binding as determinates of topical absorption, clearance and distribution. In: Riviere JE, editor. Dermal absorption models in toxicology and pharmacology. Boca Raton: CRC Press; 2006. p. 251–82.
Roberts MS, Rowland M. Hepatic elimination—dispersion model. J Pharm Sci. 1985;74:585–7.
Cross SE, Anderson C, Roberts MS. Topical penetration of commercial salicylate esters and salts using human isolated skin and clinical microdialysis studies. Br J Clin Pharmacol. 1998;46:29–35.
Cevc G, Vierl U. Spatial distribution of cutaneous microvasculature and local drug clearance after drug application on the skin. J Control Release. 2007;118:18–26.
Anissimov YG, Bracken AJ, Roberts MS. Interconnected-tubes model of hepatic elimination. J Theor Biol. 1997;188:89–101.
Leu AJ, Husmann MJ, Held T, Frisullo R, Hoffmann U, Franzeck UK. Measurement of the lymphatic clearance of the human skin using a fluorescent tracer. J Vasc Res. 2001;38:423–31.
ACKNOWLEDGMENTS
We are grateful to the financial support of the National Health & Medical Research Council of Australia and the Queensland and New South Wales Lions Medical Research Foundation. We also acknowledge Prof. Hans Schaefer’s advice in relation to experimental data in his work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anissimov, Y.G., Roberts, M.S. Modelling Dermal Drug Distribution After Topical Application in Human. Pharm Res 28, 2119–2129 (2011). https://doi.org/10.1007/s11095-011-0437-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-011-0437-2