ABSTRACT
With the increasing number of studies proposing new and optimal delivery strategies for the efficacious silencing of gene-related diseases by the local administration of siRNAs, the present review aims to provide a broad overview of the most important and latest developments of non-viral siRNA delivery systems for local administration. Moreover, the main disease targets for the local delivery of siRNA to specific tissues or organs, including the skin, the lung, the eye, the nervous system, the digestive system and the vagina, were explored.
Similar content being viewed by others
INTRODUCTION
Since Fire et al. (1) discovered that RNA interference (RNAi) is mediated by long double-stranded RNA (dsRNA) in Caenorhabditis elegans and the subsequent demonstration that RNAi, mediated by small interfering RNA (siRNA), operates in mammalian cells (2), RNAi has become a major subject of interest not only as a tool for biological research but also as an important therapeutic approach for gene-related diseases (3).
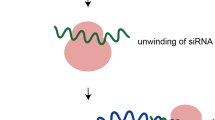
siRNA, a short double-stranded RNA that contains 21–23 nucleic acids with a 19-nucleotide duplex region, is able to inhibit the gene expression of specific proteins by a mechanism called RNA interference (4,5), which occurs in two different phases (6), as explained in Fig. 1.
Mechanisms of RNA interference. Initiation Phase: generation of effectors molecules. Nucleus: micro-RNA (miRNA) synthesis. miRNA gene is transcript by RNA Pol II/III forming miRNA primary (pri-miRNA), which is processed by Drosha and DGCR8 in miRNA precursor (pre-miRNA). pre-miRNA is exported by exportin-5 to cytoplasm. Cytoplasm: dsRNA and pre-miRNA are processed by Dicer in siRNA and miRNA, respectively. Execution Phase: incorporation of effectors molecules in protein complexes and promotion of gene silencing. siRNA or miRNA binds to RISC (RNA induced silencing complex—composed by Dicer, TRBP and Ago2). siRNA or miRNA strands are separated. Antisense strand remains bound to RISC complex, which is activated and guided to the target mRNA. The complex siRNA/RISC associates with the target mRNA promotes its degradation. The complex miRNA/RISC associates with the target mRNA promotes its degradation or translational repression, depending of the level of the complementarity.
Thus, the ability of siRNA to potently but reversibly silence genes in vivo has made siRNA particularly well-suited to be a new class of drugs that interferes with disease-causing or disease-promoting genes (7).
Specificity, potency and versatility are major advantages of RNAi therapeutics (8). Compared with small molecule drugs, siRNA has the advantage of being sequenced for highly specific inhibition of the target of interest, and genes from any molecular class can be targets. Unlike therapeutic proteins, siRNA synthesis is straightforward and does not require a cellular expression system, complex protein purification or re-folding schemes (9). Finally, compared with other gene/antisense therapies, the advantages of siRNA include its robustness, its potency (it is 10–100-fold more potent for gene silencing), its specificity of inhibition, its cytoplasmic site of action and its low risk of toxic effects (10,11).
However, despite the potential for the clinical use of siRNAs, their application to the treatment of chronic and severe diseases is limited by several factors, such as difficult cellular uptake, a low rate of cell transfection, rapid degradation by endogenous enzymes resulting in a short half-life, a negative charge that prevents the crossing of the cell membranes and insufficient bioavailability (12,13). Therefore, the in vivo effectiveness of siRNA depends on its delivery to the target tissue and the intracellular compartment of the cell type of interest within the target tissue. The first requirement is common to all classes of drugs, whereas the second one is common only for those drugs with an intracellular target (9).
In this context, several synthetic non-viral carriers have been proposed in the last few years and are quickly gaining popularity as safe and efficient vectors for delivering siRNAs to target organs. Along with the development of many innovative technologies for effective drug delivery that address the issues with the injection of siRNAs, including the use of liposomes, polymers and nanoparticles, the development of alternative routes of administration have been investigated extensively (14,15) and an increasing number of studies have proposed different approaches for the local delivery of therapeutic siRNA.
Considering that the systemic administration of siRNA faces important obstacles, including low bioavailability, systemic toxicity, rapid excretion and inefficient targeting to the affected organ or cell type (10), local administration has become an attractive and effective route, allowing the use of lower doses and reducing the side effects.
Given the increasing number of studies proposing new and optimum siRNA delivery strategies for the efficacious silencing of disease genes, the present review provides an overview of the most important and recent developments in delivering siRNA via local administration routes and discusses the main disease targets for the local delivery of siRNA to specific tissues or organs, including the skin, the lung, the eye, the nervous system, the digestive system and the vagina.
DELIVERY SYSTEMS AND METHODS TO DELIVER siRNA TOPICALLY OR LOCALLY
The widespread use of RNAi as a therapeutic approach in gene therapy depends on the delivery of nucleic acid molecules into cells and thus requires the development of clinically suitable, safe and effective drug delivery systems (16,17). In general, the ideal carrier for the local delivery of siRNA should meet several criteria.
First, the siRNA carrier should overcome the inherent barriers imposed by each administration route. For example, for cutaneous siRNA delivery, the stratum corneum, which is the outermost layer of skin, represents the primary impediment to drug skin penetration, especially for drugs with a high molecular weight and complex structure, such as siRNA (18,19). The anatomy and morphology of the lung epithelium is the main limitation for efficient pulmonary siRNA delivery (20). In addition to physical barriers in ocular siRNA therapy, such as the tear film that covers the ocular surface and the corneal and conjunctival epithelial cells, metabolic barriers may degrade and reduce the efficacy of drugs (21). Finally, an effective formulation aimed at delivering genes into the central nervous system (CNS) must cross the blood–brain barrier by accessing the endogenous receptors expressed within the membrane system (22).
The carrier should bind and condense the siRNA, providing protection against degradation, as naked siRNAs are susceptible to enzymatic degradation in the body (23). Additionally, the carrier should deliver the siRNA specifically to the target cells (24) and when in contact with these cells, it should facilitate the cellular uptake of the siRNA. In this way, the carrier will circumvent the inability of naked siRNA to passively diffuse through cellular membranes due to its relatively large molecular weight and strong anionic charge (13). Once inside the cell, the carrier should escape from endosomal trafficking to the lysosome and reach the cell cytoplasm without being metabolized (25). Finally, the carrier should release the siRNA for proper function and efficiently silence the gene (26).
Viral vectors can be applied for siRNA delivery because throughout evolution they have developed strategies to allow the entry of their genomes into the host cell. Viral vectors, such as adenoviruses and retroviruses, have high transfection efficiency but also present cytotoxicity, oncogenicity and immunogenicity (13,27,28). Alternately, non-viral vectors, such as polyplexes, lipoplexes and peptide- or protein-based systems with siRNA, have been shown to be promising tools for gene delivery mainly because they enable the incorporation of ligands for targeting specific cell types and because they are reasonably safe (13) (Fig. 2).
Schematic representation of different non-viral vectors used for siRNA delivery. Polymer-, lipid-, peptide- or protein-based systems form complexes, usually through the spontaneous electrostatic interactions, with siRNA which can be both entrapped within the core or adsorbed onto the surface of the carrier. Multifunctional nanocarriers combining several useful properties in one particle that have been developed to enhance the siRNA delivery.
Typically, cationic liposomes or cationic lipids, such as N-[1-(2,3-dioleoyloxy)]-N,N,N-trimethylammonium propane methylsulfate (DOTAP) and N-(1-(2,3-dimyristyloxypropyl)-N,N-dimethyl-(2-hydroxyethyl)ammonium bromide (DMRIE), are used to form complexes (lipoplexes) through the spontaneous electrostatic interactions between the positively charged amine head group of the cationic lipid and the phosphate group from the nucleic acid component, thereby delivering siRNA to different cell types (13,29). Most of the lipoplexes have great similarity to the cell membrane, which suggests that drug delivery occurs by membrane fusion. Although lipoplexes generally have good transfection efficiency and are easy to prepare, their poor stability and poor reproducibility should be considered (29).
The siRNA delivery systems based on cationic polymers have received attention due to their biocompatibility, their ease of production and their versatility (many physicochemical modifications can be made to obtain the intended effect) (27,29). Special interest has been given to cationic polymers that able to form nanoscale complexes (polyplexes) with siRNA based on electrostatic interactions between their positive charge and the negative charge of siRNA, which condenses the large structure of genes, neutralizes the strongly negative charge and provides good transfection rates (13,29–31).
One of the major concerns about using cationic polymers as delivery system for siRNA is toxicity due to their cationic and non-degradable characteristics. The non-degradability can be considered the main reason for the toxicity of cationic polymers, even when similar silencing activity is achieved by biodegradable and non-biodegradable polymers (31). These findings encourage the use of biodegradable and biocompatible polymer-based systems for siRNA delivery, such as the use of chitosan (CS)-based systems (32,33) and poly(lactic-co-glycolic acid) (PLGA) nanoparticles (34).
Peptide transduction domains (PTDs) or cell-penetrating peptides (CPPs) are attractive drug vectors due to their ability to translocate micro- and macromolecules across the cell membrane. These vectors can be defined as small positively charged peptides containing 10–30 amino acids that are able to interact with nucleic acids such as siRNAs by electrostatic interactions, inducing internalization through different endocytic mechanisms. Arginine and lysine residues are usually present in the structure and provide cationic charges and membrane permeability (35,36). They have also been added to previously developed siRNA vectors to optimize their effectiveness.
Table I describes different polymer-, lipid, peptide- or protein-based systems developed to deliver siRNA by local administration routes.
As shown in Table I to achieve the desired gene silencing, innovative techniques for the siRNA delivery by local administration have been proposed. The progress in pharmaceutical formulation has provided promising strategies to overcome the barriers that exist at the tissue, extracellular and cellular levels. Additionally, the elucidation of the main factors that contribute to overcome both the common and specific obstacles to the local administration of siRNA, has allowed the rational modeling of the existing delivery systems in order to optimize their effectiveness.
However, a number of challenges still confront the translation of siRNA therapy from the laboratories into the clinics, once this will depend upon the development of a system capable of providing bioactive siRNA to a specific site in a convenient dosing scheme and with a favorable toxicity profile.
In addition to the non-viral vectors described previously, physical delivery methods merit mention (Fig. 3). These may include hydrodynamic methods, gene gun, ultrasound, electroporation and iontophoresis; because the last two are the most commonly used, this review will discuss local siRNA delivery via these physical methods.
Physical methods for siRNA delivery in the skin. In the iontophoresis (a) the positively charged chamber releases the formulation with the same charge through electromigration and electroosmosis. In the gene gun (b) an adjustable low-pressure helium pulse impel the gene-coated gold particles into the target. The electroporation (c) uses electric pulses to create transient pores in a cell membrane and the ultrasound (d) alters the permeability properties of the cell membrane improving local siRNA delivery.
Electroporation, which is by far the most commonly used methodology, is a technique that consists of improving cell membrane permeability by applying an external electric field (44). This method acts by reversibly destabilizing the plasma membrane, enabling easier permeation of drugs and genetic materials (45,46). For an effective result, the obtained pores should be adequately sized; they should remain open for a sufficient amount of time, and they should not destroy membrane integrity (47). There are many advantages to the use of such technique when compared with other delivery vectors for gene therapy: (i) the transfection efficacy for primary cells is high, (ii) there are reduced safety concerns, (iii) the method is easy to apply, and (iv) there is little influence of the cell line on the efficiency (44).
Iontophoresis is another non-invasive method by which a small electric current is used to enhance the penetration of ionized drugs. In this method, the drug is applied to the surface of the electrode with the same charge, and the oppositely charged electrode is placed in another part of the body, establishing current flow. Among the notable advantages of this approach are its ease of application, its minimized systemic side effects and its increased local permeability, making iontophoresis a common technique employed for skin and ocular drug delivery (48,49).
Table II describes representative studies that propose the use of different physical methods as a strategy to improve local siRNA delivery to specific tissues or organs, such as the skin, the eye, and the nervous and digestive systems.
LOCAL DELIVERY OF siRNA: ADMINISTRATION ROUTES AND TREATMENT OF DISEASE
In addition to addressing the use of non-viral vectors in siRNA gene therapy, it is important to present the main topical administration routes and diseases that can be involved in this therapy. Skin, lung, eyes and other organs will be presented, and a link with diseases and disorders will be considered. For better understanding, the followed discussion will present the pathological aspect of the diseases, the molecular target for the siRNA approach and the highlighted studies that support the use of gene therapy with siRNA.
Skin
The skin is the largest organ of the human body and can be affected by a wide variety of diseases. For the treatment of these diseases, topical administration is advantageous because of easy accessibility to the affected regions, reduced systemic effects, the avoidance of a possible loss of therapeutic efficacy caused by first-pass metabolism, and as a non-invasive and easy-to-administer medication, topical administration improves patients’ adherence to treatment (60).
However, there remain obstacles to overcome, such as the barrier presented by the stratum corneum, the outermost layer of skin that represents the main impediment to drug penetration due to its composition and cellular distribution (18,19). As presented in the Delivery System section of the review, a strategy to resolve this issue is the development of effective, safe and clinically acceptable delivery systems and/or physical methods, allowing the penetration of drugs into the stratum corneum and thus making this attractive route of administration feasible.
The topical delivery of siRNA can strategically modulate the local expression of the genes responsible for a variety of cutaneous disorders; consequently, there is a range of potential applications for RNAi therapy for the skin. The use of siRNAs for the treatment of different skin diseases has been reported, and the results have been quite promising, as discussed below.
Allergic Skin Disease
The increased expression of CD86 in inflammatory skin diseases has been reported in humans and animals, and the blocking of its expression by siRNA influences the T cell response-specific antigen in animal models of allergic diseases. Therapy with CD86 siRNA against dendritic cells for the treatment of contact hypersensitivity and atopic dermatitis successfully reduced the innate and adaptive immune responses of the body both locally and in the regional lymph nodes. In addition, the cream-emulsion formulation used was a convenient and economical method of drug delivery (61).
Psoriasis
In contrast to allergic skin episodes, psoriasis is a chronic inflammatory disease characterized by demarcated erythematous scaly plaques. The abnormal and accelerated proliferation of keratinocytes leads to epidermal hyperplasia. Tumor necrosis factor alpha (TNF-α), a pro-inflammatory cytokine that is upregulated in psoriatic lesions, is an important molecular target for the treatment of this skin disorder. Reduction in the levels of expression of this pro-inflammatory cytokine lead to phenotypic improvements, such as reduced epidermal thickness and normalization of the skin morphology in a psoriasis animal model (62).
Given that TNF-α is known to play a pivotal role in psoriasis, Johansen et al. (63) identified other potential targets in the treatment of psoriasis, such as p38 MAPK and MAPK-activated protein kinase 2 (MK2), which posttranscriptionally regulate the expression of this and other proinflammatory cytokines. The same research group published two other reports in which they demonstrated the role of mitogen-and stress-activated protein kinase 1 (MSK1) (64) and MSK2 (65) in the pathogenesis of psoriasis, suggesting that the p38-MAPK/MSK1/MSK2 signaling pathway might constitute a potential therapeutic target for this disease.
Pachyonychia Congenita
Pachyonychia congenita is a dominant negative genetic disease of the skin caused by mutations in the genes encoding keratin. The disease is characterized by thickened nails, palmoplantar hyperkeratosis, leukokeratosis and painful keratoderma with blistering on the soles of the feet. The most frequently mutated gene is KRT6A (66–68).
Due to the locations of the lesions, non-invasive topical treatment is a great advantage of RNAi-based therapeutic technology, which selectively eliminates the functionality of the mutant allele, providing an effective treatment for this pathology. Notably, among the 24 ongoing clinical trials using siRNA, two involve the treatment of pachyonychia congenita. One of these treatments uses a specific siRNA against the KRT6A gene. This study is the first to evaluate the use of siRNA in human skin and aims to assess the safety and tolerability of intra-lesion injections in the calluses of patients with pachyonychia congenita by varying the volume and concentration of siRNA (K6a_513a.12 siRNA, known as TD101). The efficacy of the siRNA treatment was assessed through clinical examination, patient reports and experiments to quantify and distinguish the mutant keratin mRNAs (66–68).
Alopecia Areata
Alopecia areata is an autoimmune disease that affects the hair follicles, resulting in hair loss. Examination around the hair follicles revealed the infiltration of CD4+ T lymphocytes, along with a CD8+ intrafollicular infiltrate, suggesting an important role of Th1 cells in the occurrence of alopecic lesions (69). The subcutaneous injections of cationized gelatin conjugated with T-box 21 siRNA (a gene that plays an important role in Th1 cell differentiation and function) promoted the reestablishment of hair shaft elongation, confirming the importance of this mediator (37).
Wound
Wounds represent a substantial biomedical burden and are among a variety of diseases that are susceptible to the local expression of genes modulated strategically by RNAi. The mitogen activated protein kinase-1 (MAPK-1) and lamin A/C were selected as potential molecular targets because of their ubiquitous nature and their high levels of expression in cutaneous wounds. The topical application of an agarose-matrix-based system to deliver a siRNA-liposomal transfection complex was effective in silencing local gene expression in non-delimited wounds (70).
Melanoma
Melanoma is a cancer of the pigmented skin cells called melanocytes, which are localized at the epidermal-dermal junction (71). The genetic mechanisms involved in this type of cancer are not well understood; however, mutation of the protein V600EB-Raf (rapidly growing fibrosarcoma, protein member of family of serine/threonine kinases with substitution of valine to glutamic acid at the 600 position of the amino acid sequence) and the increased activity of Akt3, also known as protein kinase B, are frequent changes found in this disease (72). The employment of siRNAs specifically targeting those genes inhibited the development or growth of melanoma (50).
Rheumatoid Arthritis
Although rheumatoid arthritis is not a cutaneous disease, the skin has been extensively used as an administration route of drugs for the treatment of this disease. Osteopontin, the extracellular matrix cytokine transcribed by activated T lymphocytes, is an important therapeutic target for many inflammatory diseases, including the autoimmune disease rheumatoid arthritis, which is characterized by chronic inflammation and joint destruction. The therapeutic application of siRNA against this target was confirmed by the observed suppression of the antibody-induced development of rheumatoid arthritis symptoms by topical application of a cream formulation (GeneCream; patent pending) loaded with this silencer (73).
Lung
Due to their location and physiological function, the lungs are in contact with many pollutants and viruses, making them susceptible to many diseases, such as asthma, cancer, influenza, severe acute respiratory syndrome and tuberculosis (74,75). Lung diseases have high lethality and prevalence; thus, many studies are being conducted to find effective treatments or vaccines (76).
siRNA is a possible powerful new class of therapeutics that offers new strategies for the treatment of respiratory diseases. Moreover, because of the lung’s characteristics, such as a large and vascularized surface, local delivery to the lung through intranasal instillation or aerosol is an interesting and practical approach to the delivery of siRNA specific to that organ (75,77).
While pulmonary administration of siRNA is facilitated by negligible degradation by nucleases in the airway, siRNA delivery through the airway is not easy due to physical barriers, such as the beating of the cilia and mucociliary clearance, surface liquid that covers the airway epithelial cells and the negatively charged cell membrane surface (20,75,78). These barriers affect the efficiency of in vivo uptake of siRNA, as observed in a study conducted by Griensenbach et al. (79).
Despite these difficulties, many studies have discussed the potential targets for local delivery of siRNA to the lung, encouraging additional studies to find treatments for major lung diseases, such as cancer, influenza, severe acute respiratory syndrome and respiratory syncytial virus, as described subsequently.
Lung Cancer
Considering the incidence rates and mortality, lung cancer is one of the most common tumors worldwide. Even with increased knowledge of the genetic and molecular basis of lung cancer, most patients with non-curable tumors die in less than 12 months. Gene therapy is a well-tolerated strategy for the development of novel treatment concepts based on the inhibition of proteins that are overexpressed in tumors and may be important for invasion, growth and cell motility (77). A promising target for the treatment of cancer is the Wilms’ tumor gene 1 (WT1), whose overexpression is related to the development and progression of diverse cancers. Thus, the in vivo silencing of the WT1 gene by siRNA seems to be an effective strategy for the treatment of lung metastases (80).
Pulmonary Fibrosis
Fibrin accumulation is common in many acute and chronic pulmonary diseases, and the expression level of plasminogen activator inhibitor-1 (PAI-1) is directly correlated with the extent of lung injury-induced accumulation of collagen, a major molecule related to the development of pulmonary fibrosis. A recent study showed that intranasal administration of PAI-1-siRNA is a potential strategy to prevent the development of pulmonary fibrosis and enhance the survival rate in mice with bleomycin-induced lung injury. In addition, this is an interesting therapeutic approach that may avoid the systemic side effects associated with oral administration of a PAI-1 inhibitor (81).
Tuberculosis
Tuberculosis is a universal disease caused by infection with Mycobacterium tuberculosis that is responsible for two million deaths every year (82). The most recent candidate among the inhaled tuberculosis therapies is siRNA targeting the host chemokine XCL-1 or lymphotactin, which is known to participate in the formation of the tuberculoid granuloma (74). The treatment of mice, previously infected for 60 days with Mycobacterium tuberculosis, with aerosolized XCL1-targeting siRNA modulated this lung immunopathology (82).
Influenza
One of the most prevalent infections in humans is that of the influenza virus, which is an enveloped virus of the Orthomyxovirus family (75). Influenza infection causes up to 40,000 deaths per year in the United States. The existing vaccines are not very effective, and the use of the four drugs approved for the treatment of influenza infection is limited by side effects and the possible emergence of resistant viruses. Thus, the development of an efficient influenza therapy or vaccine is necessary. A study tested 20 siRNAs against the influenza A virus and found that specific siRNA can inhibit influenza virus production in both cell lines and embryonated chicken eggs (83).
Severe Acute Respiratory Syndrome (SARS)
SARS is a disease caused by the SARS coronavirus (SCV). Because it is a new disease, no safe and effective vaccine exists yet, though efforts toward its development have been intensified (75,84).
Using a Rhesus macaque SARS model, Li et al. (84) intranasally administered siSC2-5, a mixture of two SCV-specific siRNA duplexes, siSC2 and siSC5, that showed outstanding prophylactic and therapeutic activity in cell culture. siSC2-5 was administered prophylactically, concomitantly or post-exposure within a period of 5 days after infection, and all of the treatment regimens showed potent suppression of SCV with no toxicity in this nonhuman primate model.
Respiratory Syncytial Virus (RSV)
RSV, a member of the Pneumovirinae subfamily in the Pneumovirus genus, is an enveloped, non-segmented, negative-stranded RNA virus responsible for the most serious respiratory infections, such as bronchiolitis and pneumonia, in infants and young children (41,75). Currently, there is no vaccine to prevent RSV, and the only accepted therapy (ribavirin) is seldom used due to its teratogenicity, its limited antiviral effect, and its controversial clinical effectiveness (41,76,85).
A very interesting approach for the prevention and treatment of diseases caused by RSV is the use of siRNA therapy administered intranasally. Different studies have been conducted to validate disease targets in vivo, such as the use of siRNA against phosphoprotein (P) protein, an essential subunit of the viral RNA-dependent RNA polymerase, which reduced the pulmonary RSV titers by approximately 99.98% (76). Moreover, the application of siRNA targeting the NS1 gene in BALB/c mice before or after RSV infection significantly attenuated the disease and diminished lung inflammation, goblet cell hyperplasia and infiltration of inflammatory cells compared with control mice (41).
Another promising siRNA against RSV is ALN-RSV01, an siRNA directed against the mRNA encoding the nucleocapsid (N) protein of RSV. This protein has an important role at many critical steps in the viral replication cycle, including those involving RNA polymerase function (85). The N protein gene is among the most conserved across the various circulating RSV isolates, a characteristic that allows for a broad-spectrum activity of N-protein-targeting siRNA (86).
The in vivo studies using the siRNA against this molecular target as a therapeutic agent began with tests on animals and showed its potent antiviral effect when administered intranasally (86). In humans, De Vincenzo et al. (87) conducted safety studies with healthy volunteers and demonstrated that intranasal ALN-RSV01 administration at doses up to 150 mg daily for 5 days was well-tolerated. Later, the antiviral activity of ALN-RSV01 was tested in a randomized, double-blind, placebo-controlled trial in adults (88 subjects) experimentally infected with wild-type RSV. The study demonstrated the effectiveness of intranasal ALN-RSV01 against the inoculated virus. However, the authors suggested that further studies must be conducted to evaluate the efficiency of ALN-RSV01 in naturally infected patients with established lower respiratory tract disease (85).
Therefore, a randomized trial with 24 lung transplant patients naturally infected with RSV who received aerosolized ALN-RSV01 or placebo daily for 3 days concluded that the aerosolized ALN-RSV01 was safe and well-tolerated and that it was associated with a reduced rate of new or progressive bronchiolitis obliterans syndrome cases compared with the placebo group (88).
Eye
Ocular gene therapy has become a well-established field, given the wide variety of ocular diseases, including many that can lead to irreversible blindness and require more efficient treatments (46,89). The eye has unique features for the development of successful gene therapy. It is a relatively isolated compartment, thereby permitting local delivery, which reduces the amount of drug needed and limits the exposure to the rest of the body, thus avoiding systemic toxicity. Additionally, it is an easily accessible and immune-privileged site. Finally, the ocular diseases involving known genes enable the use of target-specific therapies, thus improving the effectiveness (46,90,91). However, an effective treatment for ocular tissues will require low drug concentrations and limited drug diffusion from the eye into the circulation (92).
There are many physical barriers that influence the release of therapeutic agents in the eye. The tear film that covers the ocular surface is a biomechanical barrier that prevents the absorption of foreign elements and permits the drainage of compounds present in the eye. The corneal and conjunctival epithelial cells are also critical barriers to overcome; the cornea has relatively poor permeability to both water-soluble and less soluble molecules, and traditionally, the role of the conjunctiva has been considered to be mainly protective, functioning as a passive physical barrier (21,93). Likewise, there is still the blood-retinal barrier to overcome (94). In addition to these physical barriers, metabolic enzymes, such as esterases, aldehyde and ketone reductases, may degrade drugs and reduce their efficacy. As a result of these anatomical and physiological constraints, topical application of a drug results in very low ocular bioavailability (21).
RNAi has been used to identify genes that promote damage in the eye and could be the basis of new treatments for many diseases, including glaucoma, age-related macular degeneration and photoreceptor degeneration (91). Historically, ophthalmology was the first area in which RNAi-based therapeutics was introduced into clinical trials (95). Given that the treatment of eye diseases is not straightforward, the use of siRNA has been constantly studied. Out of 24 clinical studies investigating siRNA, 8 are related to eye diseases (see Table III).
As showed in Table III, although there are interesting examples of clinical trials being performed with siRNA designed against molecular targets of ocular disease, which demonstrated the applicability of this therapy for different eye disorders, all of them were developed employing the naked siRNA by intravitreal injection or topical application. These may explain some clinical trials ended. Therefore, the rational design of delivery systems capable to provide these siRNA products in their active form to the eye is decisive to improve their activity, transforming them in commercial products.
Age-Related Macular Degeneration
The most common problem affecting the central regions of the choroid and retina is age-related macular degeneration, with progressive degeneration of the retinal epithelium affecting the photoreceptors and, in severe conditions, progressing to irreversible blindness (96).
Macular degeneration is triggered by the protein vascular endothelial growth factor (VEGF), which promotes blood vessel growth; the leakage of these blood vessels induces the disease. In 2004, Acuity Pharmaceuticals started the first human clinical trial with siRNAs in patients suffering from age-related macular degeneration. The VEGF-targeting siRNA (Cand5 - bevasiranib) was administered to the eye by local intravitreal injection to prevent the overgrowth of the new blood vessels. The preliminary results showed dose-related benefits. In 2006, Cand5 began clinical trials for diabetic macular edema. Shortly thereafter, Sirna Therapeutics produced their first siRNA (Sirna-027) targeting the VEGF receptor for the treatment of the same disease. A single intravitreal administration of Sirna-027 was well tolerated by patients, with improvements in visual acuity occurring in a sub-set of subjects (97,98).
However, Berkhout (99) showed evidence that the preclinical efficacy of siRNA therapeutics observed in macular degeneration-induced mouse models was most likely due to nonspecific side effects rather than sequence-specific gene knockdown. This report indicates that the development of safe siRNA-based therapies may be more challenging than anticipated and reemphasizes the importance of including appropriate controls in RNAi experiments.
Diabetic Retinopathy
Diabetic retinopathy is a leading cause of blindness for which there is currently no cure. This disease results from vascular abnormalities, including increased vascular permeability, and retinal neurodegeneration caused by irreversible changes that occur early in the course of diabetic retinopathy. Therefore, preventing the increased vascular permeability and neuronal cell death appears to be a reasonable strategy to mitigate the complications associated with this disease (100).
Oshitari et al. (101) demonstrated that abnormal expression of vascular basement membrane components may play a role in the development of the increased vascular permeability associated with diabetic retinopathy. Moreover, by screening different siRNAs (against fibronectin, laminin, or collagen IV), the authors demonstrated that the siRNA strategy may be useful in delaying or preventing excess vascular permeability.
Ocular Neovascularization
Angiogenesis from neovascularization causes several ocular diseases, including age-related macular degeneration, herpetic stromal keratitis, central and branch retina vein occlusion, trauma, various inflammatory ocular diseases and diabetic retinopathy. The exact mechanism underlying the pathogenesis of ocular neovascularization is not well understood, but it has been shown that VEGF is directly involved in new vascularization (8,102).
The possibility of using siRNA to treat neovascularization has been demonstrated by employing siRNA against VEGF or VEGF receptor 1 (VEGFR1) (103,104). Both of these therapies have been tested in clinical trials, and the studies demonstrated that topical delivery of siRNA directed against VEGF functions is efficient in suppressing corneal neovascularization (91,102).
Glaucoma
Glaucoma is a progressive optic neuropathy characterized by functional and structural impairment of the ocular tissues, including the trabecular meshwork, the optic nerve head and the retinal ganglion cells. As the disease progresses, it can cause retinal ganglion cell death, leading to irreversible blindness. The structural changes in the eye tissues lead to elevated intraocular pressure and progressive apoptotic cell death (89,96).
Glaucoma is not a single condition but a heterogeneous group of diseases that are classified according to the age of onset and the degree of ocular hypertension. The most common treatment strategy is the reduction of intraocular pressure by topical anti-glaucoma medications; however, this approach can cause serious side effects and may fail to maintain lower intraocular pressure over a period of time for a large number of patients (89,96).
Inhibition of aqueous humor secretion with RNAi-based gene therapeutic strategies has been developed. Specific siRNAs silencing gene expression in the trabecular meshwork/retina caused suppression of target gene (e.g., myocilin) expression, which might increase the conventional outflow, decrease the aqueous humor flow and promote neuroprotection. Previous studies have demonstrated that topical administration of specific siRNAs targeting carbonic anhydrase genes and alpha and beta adrenoceptors lowered intraocular pressure in rabbits at a rate comparable to that obtained using commercial products. Moreover, siRNA strategies have the advantage of producing a longer-lasting effect compared with commercial pharmaceutical products (105).
Keratitis
There are many types of keratitis, and they are classified according to the causative agent. The most frequent form of this disease is the one related to the use of contact lens and caused by Pseudomonas aeruginosa. The symptoms range from inflammatory epithelial edema to stroma infiltration, leading to corneal ulceration, stroma tissue destruction and vision loss (106).
siRNA was employed to investigate the role of two important defensins in the ocular immune defense system, murine β-defensin-1 (mBD1) and mBD2. To that end, two different mouse models (susceptible (B6) or resistant (BALB/c) to P. aeruginosa) were investigated. Knockdown experiments revealed that for host resistance against bacterial infection, the only required defensin was mBD2, which modulated the production of proinflammatory cytokines, inducible NO synthase (iNOS), TLR signaling molecules, and nuclear factor kappa B (NF-κB), that were activated in the infected cornea. Thus, this study demonstrated that the aforementioned defensin may provide a promising target for the treatment of P. aeruginosa keratitis (106).
Nervous System
The brain presents a significant challenge to biomedical science, with a notable lack of effective therapies against disorders that affect this organ. Due to its complexity, with a great diversity of cell types and variety of functions, there is a need to further understand the pathological mechanisms of brain disorders (107). In this context, therapeutics based on RNAi could help to understand pathological processes, to validate disease targets in vivo or to investigate the therapeutic potential of target genes involved in neurological disorders.
The effort required to understand the mechanisms involved in abnormal brain function and to improve delivery methods for interference technology to the CNS is justified by the vast number of diseases that affect the CNS (107–110). Neurological disorders consist of more than 600 different diseases that affect a significant portion of the population. Given that life expectancy is increasing, the occurrence of these diseases is likely to increase in the future. CNS diseases are often related to mutations, which may result in abnormal functioning resulting in a pathological state (109,110).
Some of the possible candidates for RNAi and their respective molecular targets are the following: intracranial tumors—EGFR (111); Huntington’s disease—huntingtin gene (112–115); Parkinson’s disease—PINK1 (116); neurophatic pain—P2X3 (117); neurodegenerative diseases, including amyotrophic lateral sclerosis—SOD1 (118,119) and Alzheimer’s disease—BACE1 (120); and other illnesses affected by hypoxic/ischemic events or brain inflammation—MMP9 (121,122) and c-Jun (123).
Although the RNAi technique is promising, the CNS is still a challenging region in which to deliver RNAi-based formulations because the blood–brain barrier limits the entrance of molecules and restricts the passive entrance of materials from the peripheral circulation into the brain (109).
Digestive System
The local (mucosal) administration of siRNA via endoscopic injection for the treatment of diseases affecting the gastrointestinal tract is advantageous due to the accessibility of the gastrointestinal mucosa, which allows the release of the drug directly to its site of action, thus reducing side effects and the possibility of affecting nearby tissues and organs (124).
Beyond the therapeutic use of siRNA, this technology has been employed as an important tool for elucidating the molecular mechanisms responsible for various gastrointestinal diseases and the discovery of new potential therapeutic targets, such as TNF-α for the inflammatory bowel diseases ulcerative colitis and Crohn's disease (125).
Vagina
The female reproductive mucosa is the main site of entry for many pathogens that cause infections (mainly sexually transmitted ones), inflammation and neoplastic diseases (34). The best way to prevent sexually transmitted infections is the topical application of therapeutic agents, which have the advantages of using low doses and overlapping among the primary sites of infection. Thus, the use of topically applied microbicides is very attractive as a preventive or treatment method (126).
Nevertheless, one of the main disadvantages of the topical use of such therapeutic agents is compliance because most of them should be applied immediately before sexual intercourse (43). Thus, it is important to develop therapeutic agents that are effective for a long duration after one application and can neutralize a diversity of viral strains (127).
Agents that can activate the RNAi pathway by delivering siRNA targeting specific viral and bacterial pathogens have been studied as promising microbicides, and intravaginal siRNAs could provide sustained protection against viral transmission (43,128). Overall, the studies of Palliser et al. (128) and Wu et al. (43) present interesting approaches to the delivery of siRNA into the vaginal mucosa, providing an exciting opportunity to explore the use of topically applied siRNAs for the treatment of sexually transmitted infections.
Additionally, the recent identification of siRNA targets for cervical cancer (E6, E7 and Grb10), human immunodeficiency virus (HIV) infections (CCR5) or HSV-2 infections (UL-29 AND UL-27) have increased the interest in the development of vaginal delivery systems siRNA (129).
FUTURE DIRECTIVES FOR TOPICAL siRNA DELIVERY
The consensus in the scientific community is that siRNA has emerged as one of the most promising therapeutic strategies for several diseases, especially for those with genetic causes. As described in this review, the development and use of different nanocarriers and methods have been studied for the delivery of siRNA by local administration, and important findings have been discovered. However, to maximize the potential of siRNA as therapeutic agents, strategies that allow the preferential delivery of siRNA to particular tissues and cell types and promote its action in the targeted cells have been recently developed.
The strategy of using receptor-mediated internalization seems an effective means to achieve the functional delivery of siRNA because the main pathway to internalize macromolecules, such as oligonucleotides and siRNA, is the endocytic pathway, which is subdivided into five major classes (four of which involve a cell surface receptor) (130,131).
As an example of studies conducted with this goal in mind, the use of hyaluronic acid, a natural linear polysaccharide present in the skin, lung, intestine, and extracellular matrix (132), is noteworthy. Hyaluronic acid induces receptor-mediated intracellular signaling and has been employed in different target-specific siRNA delivery systems to increase their effectiveness. Promising results have been demonstrated by combining this polysaccharide with different carriers, as the intracellular delivery of different siRNAs can be facilitated by hyaluronic acid receptor-mediated endocytosis (133,134). In the same way, a dramatic improvement in siRNA delivery to the brain was obtained by combining α-tocopherol–conjugated siRNA with high-density lipoprotein (HDL), which allows for lipoprotein receptor–mediated endocytosis (135).
Similarly, because siRNAs can be delivered into immune cells by receptor-mediated endocytosis either by encapsulating siRNAs into nanocarriers bearing targeting antibodies or ligands to cell surface receptors or by complexing siRNAs to antibody fusion proteins, a recently published study proposed an alternate approach - chimeric RNAs composed of an aptamer fused to an siRNA for targeted gene knockdown in cells bearing an aptamer-binding receptor. The CD4 aptamer-siRNA chimera was proposed to prevent HIV sexual transmission by two mechanisms of action: blocking viral entry via binding to CD4 and RNAi knockdown of viral genes, host receptors, or other host genes required for viral replication (136).
In addition to strategies for overcoming the biological barriers to the administration routes for topical siRNA delivery, the understanding of the specific mechanisms of internalization and trafficking within cells by which the nanocarriers can mediate siRNA delivery to target tissue and cells will guide the rational development of efficient nanocarriers to deliver siRNA, allowing for the topical treatment of many diseases.
CONCLUSION
Overall, the studies discussed present interesting approaches to the delivery of siRNA, providing exciting opportunities for the use of topically applied siRNAs in the treatment of a wide variety of disorders. Local delivery of siRNA avoids systemic exposure and reduces the likelihood of unexpected harmful effects elsewhere in the body as a result of the drug. The successful delivery systems examples, either in vivo or clinical studies, presented in this review, show the complexity of the formulation design, which depends on several factors, including chemical interactions between siRNA and formulation components, technological process for obtainment of delivery systems and specific siRNAs, strategies to overcome different physiological barriers of topical/local administration route. The peculiarities of the different local administration route do not allow stating an ideal delivery system for siRNA; however, this review brings information that can conduce further studies in a rational way. The widespread use of RNAi therapeutics for disease prevention and treatment will depend on the multidisciplinary application of the pharmaceutical technology, that allow the construction of “smart and multifunctional” delivery systems along with the elucidation of new molecular targets and sequence-specific siRNA that block such targets. Therefore, despite the remarkable developments made in this area during the last decade, improving local siRNA application remains a significant research area and clearly warrants further studies.
REFERENCES
Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–11.
Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–8.
Geusens B, Sanders N, Prow T, Van Gele M, Lambert J. Cutaneous short-interfering RNA therapy. Expert Opin Drug Deliv. 2009;6:1333–49.
David S, Pitard B, Benoit JP, Passirani C. Non-viral nanosystems for systemic siRNA delivery. Pharmacol Res. 2010;62:100–14.
Kim HK, Davaa E, Myung CS, Park JS. Enhanced siRNA delivery using cationic liposomes with new polyarginine-conjugated PEG-lipid. Int J Pharm. 2010;392:141–7.
De Paula D, Bentley MV, Mahato RI. Hydrophobization and bioconjugation for enhanced siRNA delivery and targeting. RNA. 2007;13:431–56.
de Fougerolles AR. Delivery vehicles for small interfering RNA in vivo. Hum Gene Ther. 2008;19:125–32.
Uprichard SL. The therapeutic potential of RNA interference. FEBS Lett. 2005;579:5996–6007.
Sah DW. Therapeutic potential of RNA interference for neurological disorders. Life Sci. 2006;79:1773–80.
Durcan N, Murphy C, Cryan SA. Inhalable siRNA: potential as a therapeutic agent in the lungs. Mol Pharm. 2008;5:559–66.
Ozpolat B, Sood AK, Lopez-Berestein G. Nanomedicine based approaches for the delivery of siRNA in cancer. J Intern Med. 2010;267:44–53.
Nimesh S, Chandra R. Polyethylenimine nanoparticles as an efficient in vitro siRNA delivery system. Eur J Pharm Biopharm. 2009;73:43–9.
Reischl D, Zimmer A. Drug delivery of siRNA therapeutics: potentials and limits of nanosystems. Nanomedicine. 2009;5:8–20.
Fattal E, Bochot A. State of the art and perspectives for the delivery of antisense oligonucleotides and siRNA by polymeric nanocarriers. Int J Pharm. 2008;364:237–48.
Wu SY, McMillan NA. Lipidic systems for in vivo siRNA delivery. AAPS J. 2009;11:639–52.
Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8:129–38.
Schafer J, Hobel S, Bakowsky U, Aigner A. Liposome-polyethylenimine complexes for enhanced DNA and siRNA delivery. Biomaterials. 2010;31:6892–900.
Madison KC. Barrier function of the skin: “la raison d’etre” of the epidermis. J Investig Dermatol. 2003;121:231–41.
Cevc G, Vierl U. Nanotechnology and the transdermal route: a state of the art review and critical appraisal. J Control Release. 2010;141:277–99.
Gutbier B, Kube SM, Reppe K, Santel A, Lange C, Kaufmann J, et al. RNAi-mediated suppression of constitutive pulmonary gene expression by small interfering RNA in mice. Pulm Pharmacol Ther. 2010;23:334–44.
de la Fuente M, Ravina M, Paolicelli P, Sanchez A, Seijo B, Alonso MJ. Chitosan-based nanostructures: a delivery platform for ocular therapeutics. Adv Drug Deliv Rev. 2010;62:100–17.
Son S, Hwang do W, Singha K, Jeong JH, Park TG, Lee DS, et al. RVG peptide tethered bioreducible polyethylenimine for gene delivery to brain. J Control Release. 2010;155:18–25.
Pittella F, Zhang M, Lee Y, Kim HJ, Tockary T, Osada K, et al. Enhanced endosomal escape of siRNA-incorporating hybrid nanoparticles from calcium phosphate and PEG-block charge-conversional polymer for efficient gene knockdown with negligible cytotoxicity. Biomaterials. 2011;32:3106–14.
Davidson BL, Paulson HL. Molecular medicine for the brain: silencing of disease genes with RNA interference. Lancet Neurol. 2004;3:145–9.
Dehousse V, Garbacki N, Colige A, Evrard B. Development of pH-responsive nanocarriers using trimethylchitosans and methacrylic acid copolymer for siRNA delivery. Biomaterials. 2010;31:1839–49.
Fernandez CA, Rice KG. Engineered nanoscaled polyplex gene delivery systems. Mol Pharm. 2009;6:1277–89.
Ghosn B, Singh A, Li M, Vlassov AV, Burnett C, Puri N, et al. Efficient gene silencing in lungs and liver using imidazole-modified chitosan as a nanocarrier for small interfering RNA. Oligonucleotides. 2010;20:163–72.
Xiong XB, Uludag H, Lavasanifar A. Virus-mimetic polymeric micelles for targeted siRNA delivery. Biomaterials. 2010;31:5886–93.
Lam JK, Liang W, Chan HK. Pulmonary delivery of therapeutic siRNA. Adv Drug Deliv Rev. 2012;64:1–14.
Krebs MD, Alsberg E. Localized, targeted, and sustained siRNA delivery. Chemistry. 2011;17:3054–62.
Varkouhi AK, Lammers T, Schiffelers RM, van Steenbergen MJ, Hennink WE, Storm G. Gene silencing activity of siRNA polyplexes based on biodegradable polymers. Eur J Pharm Biopharm. 2011;77:450–7.
Oh YK, Park TG. siRNA delivery systems for cancer treatment. Adv Drug Deliv Rev. 2009;61:850–62.
Mao S, Sun W, Kissel T. Chitosan-based formulations for delivery of DNA and siRNA. Adv Drug Deliv Rev. 2010;62:12–27.
Woodrow KA, Cu Y, Booth CJ, Saucier-Sawyer JK, Wood MJ, Saltzman WM. Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nat Mater. 2009;8:526–33.
Meade BR, Dowdy SF. Enhancing the cellular uptake of siRNA duplexes following noncovalent packaging with protein transduction domain peptides. Adv Drug Deliv Rev. 2008;60:530–6.
Patlolla RR, Desai PR, Belay K, Singh MS. Translocation of cell penetrating peptide engrafted nanoparticles across skin layers. Biomaterials. 2010;31:5598–607.
Nakamura M, Jo J, Tabata Y, Ishikawa O. Controlled delivery of T-box21 small interfering RNA ameliorates autoimmune alopecia (Alopecia Areata) in a C3H/HeJ mouse model. Am J Pathol. 2008;172:650–8.
Hsu T, Mitragotri S. Delivery of siRNA and other macromolecules into skin and cells using a peptide enhancer. Proc Natl Acad Sci U S A. 2011;108:15816–21.
Vicentini FT, Depieri LV, Polizello AC, Ciampo JO, Spadaro AC, Fantini MC, Bentley MV. Liquid crystalline phase nanodispersions enable skin delivery of siRNA. Eur J Pharm Biopharm. 2013;83:16–24.
Hatakeyama H, Ito E, Akita H, Oishi M, Nagasaki Y, Futaki S, et al. A pH-sensitive fusogenic peptide facilitates endosomal escape and greatly enhances the gene silencing of siRNA-containing nanoparticles in vitro and in vivo. J Control Release. 2009;139:127–32.
Zhang W, Yang H, Kong X, Mohapatra S, San Juan-Vergara H, Hellermann G, et al. Inhibition of respiratory syncytial virus infection with intranasal siRNA nanoparticles targeting the viral NS1 gene. Nat Med. 2005;11:56–62.
Kim ID, Lim CM, Kim JB, Nam HY, Nam K, Kim SW, et al. Neuroprotection by biodegradable PAMAM ester (e-PAM-R)-mediated HMGB1 siRNA delivery in primary cortical cultures and in the postischemic brain. J Control Release. 2010;142:422–30.
Wu Y, Navarro F, Lal A, Basar E, Pandey RK, Manoharan M, et al. Durable protection from Herpes Simplex Virus-2 transmission following intravaginal application of siRNAs targeting both a viral and host gene. Cell Host Microbe. 2009;5:84–94.
Geng T, Zhan Y, Wang HY, Witting SR, Cornetta KG, Lu C. Flow-through electroporation based on constant voltage for large-volume transfection of cells. J Control Release. 2010;144:91–100.
Bejjani RA, Andrieu C, Bloquel C, Berdugo M, BenEzra D, Behar-Cohen F. Electrically assisted ocular gene therapy. Surv Ophthalmol. 2007;52:196–208.
Conley SM, Naash MI. Nanoparticles for retinal gene therapy. Prog Retin Eye Res. 2010;29:376–97.
Talele S, Gaynor P, Cree MJ, van Ekeran J. Modelling single cell electroporation with bipolar pulse parameters and dynamic pore radii. J Electrost. 2010;68:261–74.
Eljarrat-Binstockand E, Domb AJ. Iontophoresis: a non-invasive ocular drug delivery. J Control Release. 2006;110:479–89.
Tesselaar E, Sjoberg F. Transdermal iontophoresis as an in-vivo technique for studying microvascular physiology. Microvasc Res. 2010;81:88–96.
Tran MA, Gowda R, Sharma A, Park EJ, Adair J, Kester M, et al. Targeting V600EB-Raf and Akt3 using nanoliposomal-small interfering RNA inhibits cutaneous melanocytic lesion development. Cancer Res. 2008;68:7638–49.
Lee WR, Shen SC, Zhuo RZ, Wang KC, Fang JY. Enhancement of topical small interfering RNA delivery and expression by low-fluence erbium:YAG laser pretreatment of skin. Hum Gene Ther. 2009;20:580–8.
Kigasawa K, Kajimoto K, Hama S, Saito A, Kanamura K, Kogure K. Noninvasive delivery of siRNA into the epidermis by iontophoresis using an atopic dermatitis-like model rat. Int J Pharm. 2010;383:157–60.
Inoue T, Sugimoto M, Sakurai T, Saito R, Futaki N, Hashimoto Y, et al. Modulation of scratching behavior by silencing an endogenous cyclooxygenase-1 gene in the skin through the administration of siRNA. J Gene Med. 2007;9:994–1001.
Hao J, Li SK, Liu CY, Kao WW. Electrically assisted delivery of macromolecules into the corneal epithelium. Exp Eye Res. 2009;89:934–41.
Prechtel AT, Turza NM, Theodoridis AA, Kummer M, Steinkasserer A. Small interfering RNA (siRNA) delivery into monocyte-derived dendritic cells by electroporation. J Immunol Methods. 2006;311:139–52.
Jantsch J, Turza N, Volke M, Eckardt KU, Hensel M, Steinkasserer A, et al. Small interfering RNA (siRNA) delivery into murine bone marrow-derived dendritic cells by electroporation. J Immunol Methods. 2008;337:71–7.
Boudes M, Pieraut S, Valmier J, Carroll P, Scamps F. Single-cell electroporation of adult sensory neurons for gene screening with RNA interference mechanism. J Neurosci Methods. 2008;170:204–11.
Tanaka M, Yanagawa Y, Hirashima N. Transfer of small interfering RNA by single-cell electroporation in cerebellar cell cultures. J Neurosci Methods. 2009;178:80–6.
Ghartey-Tagoe EB, Babbin BA, Nusrat A, Neish AS, Prausnitz MR. Plasmid DNA and siRNA transfection of intestinal epithelial monolayers by electroporation. Int J Pharm. 2006;315:122–33.
Prausnitz MR, Mitragotri S, Langer R. Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov. 2004;3:115–24.
Ritprajak P, Hashiguchi M, Azuma M. Topical application of cream-emulsified CD86 siRNA ameliorates allergic skin disease by targeting cutaneous dendritic cells. Mol Ther. 2008;16:1323–30.
Jakobsen M, Stenderup K, Rosada C, Moldt B, Kamp S, Dam TN, et al. Amelioration of psoriasis by anti-TNF-alpha RNAi in the xenograft transplantation model. Mol Ther. 2009;17:1743–53.
Johansen C, Funding AT, Otkjaer K, Kragballe K, Jensen UB, Madsen M, et al. Protein expression of TNF-alpha in psoriatic skin is regulated at a posttranscriptional level by MAPK-activated protein kinase 2. J Immunol. 2006;176:1431–8.
Funding AT, Johansen C, Kragballe K, Otkjaer K, Jensen UB, Madsen MW, et al. Mitogen- and stress-activated protein kinase 1 is activated in lesional psoriatic epidermis and regulates the expression of pro-inflammatory cytokines. J Investig Dermatol. 2006;126:1784–91.
Funding AT, Johansen C, Kragballe K, Iversen L. Mitogen- and stress-activated protein kinase 2 and cyclic AMP response element binding protein are activated in lesional psoriatic epidermis. J Investig Dermatol. 2007;127:2012–9.
Hickerson RP, Leachman SA, Pho LN, Gonzalez-Gonzalez E, Smith FJ, McLean WH, et al. Development of quantitative molecular clinical end points for siRNA clinical trials. J Investig Dermatol. 2011;131:1029–36.
Leachman SA, Hickerson RP, Hull PR, Smith FJ, Milstone LM, Lane EB, et al. Therapeutic siRNAs for dominant genetic skin disorders including pachyonychia congenita. J Dermatol Sci. 2008;51:151–7.
Leachman SA, Hickerson RP, Schwartz ME, Bullough EE, Hutcherson SL, Boucher KM, et al. First-in-human mutation-targeted siRNA phase Ib trial of an inherited skin disorder. Mol Ther. 2010;18:442–6.
Gilhar A, Kalish RS. Alopecia areata: a tissue specific autoimmune disease of the hair follicle. Autoimmun Rev. 2006;5:64–9.
Thanik VD, Greives MR, Lerman OZ, Seiser N, Dec W, Chang CC, et al. Topical matrix-based siRNA silences local gene expression in a murine wound model. Gene Ther. 2007;14:1305–8.
Hsu MY, Meier F, Herlyn M. Melanoma development and progression: a conspiracy between tumor and host. Differentiation. 2002;70:522–36.
Fedorenko IV, Paraiso KH, Smalley KS. Acquired and intrinsic BRAF inhibitor resistance in BRAF V600E mutant melanoma. Biochem Pharmacol. 2011;82:201–9.
Takanashi M, Oikawa K, Sudo K, Tanaka M, Fujita K, Ishikawa A, et al. Therapeutic silencing of an endogenous gene by siRNA cream in an arthritis model mouse. Gene Ther. 2009;16:982–9.
Misra A, Hickey AJ, Rossi C, Borchard G, Terada H, Makino K, et al. Inhaled drug therapy for treatment of tuberculosis. Tuberculosis (Edinb). 2010;91:71–81.
Thomas M, Lu JJ, Chen J, Klibanov AM. Non-viral siRNA delivery to the lung. Adv Drug Deliv Rev. 2007;59:124–33.
Bitko V, Musiyenko A, Shulyayeva O, Barik S. Inhibition of respiratory viruses by nasally administered siRNA. Nat Med. 2005;11:50–5.
Gunther M, Lipka J, Malek A, Gutsch D, Kreyling W, Aigner A. Polyethylenimines for RNAi-mediated gene targeting in vivo and siRNA delivery to the lung. Eur J Pharm Biopharm. 2010;77:438–49.
Rosenecker J, Naundorf S, Gersting SW, Hauck RW, Gessner A, Nicklaus P, et al. Interaction of bronchoalveolar lavage fluid with polyplexes and lipoplexes: analysing the role of proteins and glycoproteins. J Gene Med. 2003;5:49–60.
Griesenbach U, Kitson C, Escudero Garcia S, Farley R, Singh C, Somerton L, et al. Inefficient cationic lipid-mediated siRNA and antisense oligonucleotide transfer to airway epithelial cells in vivo. Respir Res. 2006;7:26.
Zamora-Avila DE, Zapata-Benavides P, Franco-Molina MA, Saavedra-Alonso S, Trejo-Avila LM, Resendez-Perez D, et al. WT1 gene silencing by aerosol delivery of PEI-RNAi complexes inhibits B16-F10 lung metastases growth. Cancer Gene Ther. 2009;16:892–9.
Senoo T, Hattori N, Tanimoto T, Furonaka M, Ishikawa N, Fujitaka K, et al. Suppression of plasminogen activator inhibitor-1 by RNA interference attenuates pulmonary fibrosis. Thorax. 2010;65:334–40.
Rosas-Taraco AG, Higgins DM, Sanchez-Campillo J, Lee EJ, Orme IM, Gonzalez-Juarrero M. Intrapulmonary delivery of XCL1-targeting small interfering RNA in mice chronically infected with Mycobacterium tuberculosis. Am J Respir Cell Mol Biol. 2009;41:136–45.
Ge Q, McManus MT, Nguyen T, Shen CH, Sharp PA, Eisen HN, et al. RNA interference of influenza virus production by directly targeting mRNA for degradation and indirectly inhibiting all viral RNA transcription. Proc Natl Acad Sci U S A. 2003;100:2718–23.
Li BJ, Tang Q, Cheng D, Qin C, Xie FY, Wei Q, et al. Using siRNA in prophylactic and therapeutic regimens against SARS coronavirus in Rhesus macaque. Nat Med. 2005;11:944–51.
DeVincenzo J, Lambkin-Williams R, Wilkinson T, Cehelsky J, Nochur S, Walsh E, et al. A randomized, double-blind, placebo-controlled study of an RNAi-based therapy directed against respiratory syncytial virus. Proc Natl Acad Sci U S A. 2010;107:8800–5.
Alvarez R, Elbashir S, Borland T, Toudjarska I, Hadwiger P, John M, et al. RNA interference-mediated silencing of the respiratory syncytial virus nucleocapsid defines a potent antiviral strategy. Antimicrob Agents Chemother. 2009;53:3952–62.
DeVincenzo J, Cehelsky JE, Alvarez R, Elbashir S, Harborth J, Toudjarska I, et al. Evaluation of the safety, tolerability and pharmacokinetics of ALN-RSV01, a novel RNAi antiviral therapeutic directed against respiratory syncytial virus (RSV). Antiviral Res. 2008;77:225–31.
Zamora MR, Budev M, Rolfe M, Gottlieb J, Humar A, Devincenzo J, et al. RNA interference therapy in lung transplant patients infected with respiratory syncytial virus. Am J Respir Crit Care Med. 2010;183:531–8.
Clark AF, Yorio T. Ophthalmic drug discovery. Nat Rev Drug Discov. 2003;2:448–59.
Borras T. Recent developments in ocular gene therapy. Exp Eye Res. 2003;76:643–52.
Campochiaro PA. Potential applications for RNAi to probe pathogenesis and develop new treatments for ocular disorders. Gene Ther. 2006;13:559–62.
Bloquel C, Bourges JL, Touchard E, Berdugo M, BenEzra D, Behar-Cohen F. Non-viral ocular gene therapy: potential ocular therapeutic avenues. Adv Drug Deliv Rev. 2006;58:1224–42.
Lee TW, Robinson JR. Drug delivery to the posterior segment of the eye IV: theoretical formulation of a drug delivery system for subconjunctival injection. J Ocul Pharmacol Ther. 2009;25:29–37.
Bodor N, Buchwald P. Ophthalmic drug design based on the metabolic activity of the eye: soft drugs and chemical delivery systems. AAPS J. 2005;7:E820–33.
Nguyen T, Menocal EM, Harborth J, Fruehauf JH. RNAi therapeutics: an update on delivery. Curr Opin Mol Ther. 2008;10:158–67.
Naik R, Mukhopadhyay A, Ganguli M. Gene delivery to the retina: focus on non-viral approaches. Drug Discov Today. 2009;14:306–15.
Akhtar S, Benter I. Toxicogenomics of non-viral drug delivery systems for RNAi: potential impact on siRNA-mediated gene silencing activity and specificity. Adv Drug Deliv Rev. 2007;59:164–82.
Del Amo EM, Urtti A. Current and future ophthalmic drug delivery systems. A shift to the posterior segment. Drug Discov Today. 2008;13:135–43.
Berkhout B. An eye-opener for RNAi therapeutics. J Formos Med Assoc. 2008;107:749–50.
Oshitari T. Non-viral gene therapy for diabetic retinopathy. Drug Dev Res. 2006;67:835–41.
Oshitari T, Brown D, Roy S. SiRNA strategy against overexpression of extracellular matrix in diabetic retinopathy. Exp Eye Res. 2005;81:32–7.
Zhang SX, Ma JX. Ocular neovascularization: implication of endogenous angiogenic inhibitors and potential therapy. Prog Retin Eye Res. 2007;26:1–37.
Kim B, Tang Q, Biswas PS, Xu J, Schiffelers RM, Xie FY, et al. Inhibition of ocular angiogenesis by siRNA targeting vascular endothelial growth factor pathway genes: therapeutic strategy for herpetic stromal keratitis. Am J Pathol. 2004;165:2177–85.
Shen J, Samul R, Silva RL, Akiyama H, Liu H, Saishin Y, et al. Suppression of ocular neovascularization with siRNA targeting VEGF receptor 1. Gene Ther. 2006;13:225–34.
Liu X, Rasmussen CA, Gabelt BT, Brandt CR, Kaufman PL. Gene therapy targeting glaucoma: where are we? Surv Ophthalmol. 2009;54:472–86.
Wu M, McClellan SA, Barrett RP, Hazlett LD. Beta-defensin-2 promotes resistance against infection with P. aeruginosa. J Immunol. 2009;182:1609–16.
Roy I, Stachowiak MK, Bergey EJ. Nonviral gene transfection nanoparticles: function and applications in the brain. Nanomedicine. 2008;4:89–97.
Thakker DR, Hoyer D, Cryan JF. Interfering with the brain: use of RNA interference for understanding the pathophysiology of psychiatric and neurological disorders. Pharmacol Ther. 2006;109:413–38.
Cazzin C, Ring CJ. Recent advances in the manipulation of murine gene expression and its utility for the study of human neurological disease. Biochim Biophys Acta. 2010;1802:796–807.
Boudreau RL, Davidson BL. RNAi therapeutics for CNS disorders. Brain Res. 2010;1338:112–21.
Boado RJ. RNA interference and nonviral targeted gene therapy of experimental brain cancer. NeuroRx. 2005;2:139–50.
Harper SQ, Staber PD, He X, Eliason SL, Martins IH, Mao Q, et al. RNA interference improves motor and neuropathological abnormalities in a Huntington’s disease mouse model. Proc Natl Acad Sci U S A. 2005;102:5820–5.
Rodriguez-Lebron E, Denovan-Wright EM, Nash K, Lewin AS, Mandel RJ. Intrastriatal rAAV-mediated delivery of anti-huntingtin shRNAs induces partial reversal of disease progression in R6/1 Huntington’s disease transgenic mice. Mol Ther. 2005;12:618–33.
Denovan-Wright EM, Rodriguez-Lebron E, Lewin AS, Mandel RJ. Unexpected off-targeting effects of anti-huntingtin ribozymes and siRNA in vivo. Neurobiol Dis. 2008;29:446–55.
Lombardi MS, Jaspers L, Spronkmans C, Gellera C, Taroni F, Di Maria E, et al. A majority of Huntington’s disease patients may be treatable by individualized allele-specific RNA interference. Exp Neurol. 2009;217:312–9.
Deng H, Jankovic J, Guo Y, Xie W, Le W. Small interfering RNA targeting the PINK1 induces apoptosis in dopaminergic cells SH-SY5Y. Biochem Biophys Res Commun. 2005;337:1133–8.
Dorn G, Patel S, Wotherspoon G, Hemmings-Mieszczak M, Barclay J, Natt FJ, et al. siRNA relieves chronic neuropathic pain. Nucleic Acids Res. 2004;32(e49).
Ralph GS, Radcliffe PA, Day DM, Carthy JM, Leroux MA, Lee DC, et al. Silencing mutant SOD1 using RNAi protects against neurodegeneration and extends survival in an ALS model. Nat Med. 2005;11:429–33.
Raoul C, Abbas-Terki T, Bensadoun JC, Guillot S, Haase G, Szulc J, et al. Lentiviral-mediated silencing of SOD1 through RNA interference retards disease onset and progression in a mouse model of ALS. Nat Med. 2005;11:423–8.
Singer O, Marr RA, Rockenstein E, Crews L, Coufal NG, Gage FH, et al. Targeting BACE1 with siRNAs ameliorates Alzheimer disease neuropathology in a transgenic model. Nat Neurosci. 2005;8:1343–9.
Hu Q, Chen C, Yan J, Yang X, Shi X, Zhao J, et al. Therapeutic application of gene silencing MMP-9 in a middle cerebral artery occlusion-induced focal ischemia rat model. Exp Neurol. 2009;216:35–46.
Bonoiu A, Mahajan SD, Ye L, Kumar R, Ding H, Yong KT, et al. MMP-9 gene silencing by a quantum dot-siRNA nanoplex delivery to maintain the integrity of the blood brain barrier. Brain Res. 2009;1282:142–55.
Cardoso AL, Costa P, de Almeida LP, Simoes S, Plesnila N, Culmsee C, et al. Tf-lipoplex-mediated c-Jun silencing improves neuronal survival following excitotoxic damage in vivo. J Control Release. 2010;142:392–403.
Pellish RS, Nasir A, Ramratnam B, Moss SF. Review article: RNA interference–potential therapeutic applications for the gastroenterologist. Aliment Pharmacol Ther. 2008;27:715–23.
Zhang Y, Cristofaro P, Silbermann R, Pusch O, Boden D, Konkin T, et al. Engineering mucosal RNA interference in vivo. Mol Ther. 2006;14:336–42.
Rossi JJ. Cholesterol paves the way for topically applied viricides. Cell Host Microbe. 2009;5:6–7.
Cristofaro P, Ramratnam B. RNAi tackles a sexually transmitted disease. Nat Biotechnol. 2006;24:48–9.
Palliser D, Chowdhury D, Wang QY, Lee SJ, Bronson RT, Knipe DM, et al. An siRNA-based microbicide protects mice from lethal herpes simplex virus 2 infection. Nature. 2006;439:89–94.
Wu SY, Chang HI, Burgess M, McMillan NA. Vaginal delivery of siRNA using a novel PEGylated lipoplex-entrapped alginate scaffold system. J Control Release. 2011;155:418–26.
Juliano R, Bauman J, Kang H, Ming X. Biological barriers to therapy with antisense and siRNA oligonucleotides. Mol Pharm. 2009;6:686–95.
Ming X. Cellular delivery of siRNA and antisense oligonucleotides via receptor-mediated endocytosis. Expert Opin Drug Deliv. 2011;8:435–49.
Jiang G, Park K, Kim J, Kim KS, Oh EJ, Kang H, et al. Hyaluronic acid-polyethyleneimine conjugate for target specific intracellular delivery of siRNA. Biopolymers. 2008;89:635–42.
Jiang G, Park K, Kim J, Kim KS, Hahn SK. Target specific intracellular delivery of siRNA/PEI-HA complex by receptor mediated endocytosis. Mol Pharm. 2009;6:727–37.
Shen Y, Wang B, Lu Y, Ouahab A, Li Q, Tu J. A novel tumor-targeted delivery system with hydrophobized hyaluronic acid-spermine conjugates (HHSCs) for efficient receptor-mediated siRNA delivery. Int J Pharm. 2011;414:233–43.
Uno Y, Piao W, Miyata K, Nishina K, Mizusawa H, Yokota T. High-density lipoprotein facilitates in vivo delivery of alpha-tocopherol-conjugated short-interfering RNA to the brain. Hum Gene Ther. 2011;22:711–9.
Wheeler LA, Trifonova R, Vrbanac V, Basar E, McKernan S, Xu Z, et al. Inhibition of HIV transmission in human cervicovaginal explants and humanized mice using CD4 aptamer-siRNA chimeras. J Clin Invest. 2011;121:2401–12.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vicentini, F.T.M.d.C., Borgheti-Cardoso, L.N., Depieri, L.V. et al. Delivery Systems and Local Administration Routes for Therapeutic siRNA. Pharm Res 30, 915–931 (2013). https://doi.org/10.1007/s11095-013-0971-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-013-0971-1