Abstract
Purpose
The present study explores the potential of bicontinous cubic liquid crystalline nanoparticles (LCNPs) for improving therapeutic potential of doxorubicin.
Methods
Phytantriol based Dox-LCNPs were prepared using hydrotrope method, optimized for various formulation components, process variables and lyophilized. Structural elucidation of the reconstituted formulation was performed using HR-TEM and SAXS analysis. The developed formulation was subjected to exhaustive cell culture experiments for delivery potential (Caco-2 cells) and efficacy (MCF-7 cells). Finally, in vivo pharmacokinetics, pharmacodynamic studies in DMBA induced breast cancer model and cardiotoxicity were also evaluated.
Results
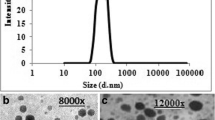
The reconstituted formulation exhibited Pn3m type cubic structure, evident by SAXS and posed stability in simulated gastrointestinal fluids and at accelerated stability conditions for 6 months. Dox-LCNPs revealed significantly higher cell cytotoxicity (16.23-fold) against MCF-7 cell lines as compared to free drug owing to its preferential localization in the vicinity of nucleus. Furthermore, Caco-2 cell experiments revealed formation of reversible “virtual pathways” in the cell membrane for Dox-LCNPs and hence posed significantly higher relative oral bioavailability (17.74-fold). Subsequently, Single dose of Dox-LCNPs (per oral) led to significant reduction in % tumor burden (~42%) as compared that of ~31% observed in case of Adriamycin® (i.v.) when evaluated in DMBA induced breast cancer model. Moreover, Dox induced cardiotoxicity was also found to be significantly lower in case of Dox-LCNPs as compared to clinical formulations (Adriamycin® and Lipodox®).
Conclusion
Incorporation of Dox in the novel LCNPs demonstrated improved antitumor efficacy and safety profile and can be a viable option for oral chemotherapy.











Similar content being viewed by others
References
Rahman A, Carmichael D, Harris M, Roh JK. Comparative pharmacokinetics of free doxorubicin and doxorubicin entrapped in cardiolipin liposomes. Cancer Res. 1986;46(5):2295–9.
Gordon KB, Tajuddin A, Guitart J, Kuzel TM, Eramo LR, VonRoenn J. Hand-foot syndrome associated with liposome-encapsulated doxorubicin therapy. Cancer. 1995;75(8):2169–73.
Ryberg M, Nielsen D, Skovsgaard T, Hansen J, Jensen BV, Dombernowsky P. Epirubicin cardiotoxicity: an analysis of 469 patients with metastatic breast cancer. J Clin Oncol. 1998;16(11):3502–8.
Beijnen J, Van der Houwen O, Underberg W. Aspects of the degradation kinetics of doxorubicin in aqueous solution. Int J Pharm. 1986;32(2):123–31.
Jain AK, Swarnakar NK, Das M, Godugu C, Singh RP, Rao PR, et al. Augmented anticancer efficacy of doxorubicin-loaded polymeric nanoparticles after oral administration in a breast cancer induced animal model. Mol Pharm. 2011;8(4):1140–51.
Thanki K, Gangwal RP, Sangamwar AT, Jain S. Oral delivery of anticancer drugs: challenges and opportunities. J Control Release. 2013;170(1):15–40.
Kalaria DR, Sharma G, Beniwal V, Ravi Kumar MNV. Design of biodegradable nanoparticles for oral delivery of doxorubicin: in vivo pharmacokinetics and toxicity studies in rats. Pharm Res. 2009;26(3):492–501.
Jain S, Patil SR, Swarnakar NK, Agrawal AK. Oral delivery of doxorubicin using novel polyelectrolyte-stabilized liposomes (layersomes). Mol Pharm. 2012;9(9):2626–35.
Guo C, Wang J, Cao F, Lee RJ, Zhai G. Lyotropic liquid crystal systems in drug delivery. Drug Discov Today. 2010;15(23–24):1032–40.
Barauskas J, Johnsson M, Tiberg F. Self-assembled lipid superstructures: beyond vesicles and liposomes. Nano Lett. 2005;5(8):1615.
Yang D, Armitage B, Marder SR. Cubic liquid-crystalline nanoparticles. Angew Chem Int Ed Engl. 2004;43(34):4402–9.
Lian R, Lu Y, Qi J, Tan Y, Niu M, Guan P, et al. Silymarin glyceryl monooleate/poloxamer 407 liquid crystalline matrices: physical characterization and enhanced oral bioavailability. AAPS PharmSciTech. 2011;12(4):1234–40.
Lai J, Chen J, Lu Y, Sun J, Hu F, Yin Z, et al. Glyceryl monooleate/poloxamer 407 cubic nanoparticles as oral drug delivery systems: I. In vitro evaluation and enhanced oral bioavailability of the poorly water-soluble drug simvastatin. AAPS Pharm Sci Technol. 2009;10(3):960–6.
Tamayo-Esquivel D, Ganem-Quintanar A, Martinez AL, Navarrete-Rodriguez M, Rodriguez-Romo S, Quintanar-Guerrero D. Evaluation of the enhanced oral effect of omapatrilat-monolein nanoparticles prepared by the emulsification-diffusion method. J Nanosci Nanotechnol. 2006;6(9–10):3134–8.
Nguyen TH, Hanley T, Porter CJ, Larson I, Boyd BJ. Phytantriol and glyceryl monooleate cubic liquid crystalline phases as sustained-release oral drug delivery systems for poorly water-soluble drugs II. In-vivo evaluation. J Pharm Pharmacol. 2010;62(7):856–65.
Nguyen TH, Hanley T, Porter CJ, Larson I, Boyd BJ. Phytantriol and glyceryl monooleate cubic liquid crystalline phases as sustained-release oral drug delivery systems for poorly water soluble drugs I. Phase behaviour in physiologically-relevant media. J Pharm Pharmacol. 2010;62(7):844–55.
Nguyen TH, Hanley T, Porter CJ, Boyd BJ. Nanostructured liquid crystalline particles provide long duration sustained-release effect for a poorly water soluble drug after oral administration. J Control Release. 2011;153(2):180–6.
Final report on the safety assessment of phytantriol1. Int J Toxicol. 2007;26(1):107–14.
Lindström M, Ljusberg-Wahren H, Larsson K, Borgström B. Aqueous lipid phases of relevance to intestinal fat digestion and absorption. Lipids. 1981;16(10):749–54.
US Department of Agriculture. Glycerol monooleate processing. http://www.ams.usda.gov/AMSv1.0/getfile?dDocName=STELPRDC5057603 (accessed on 12 Sep, 2013).
Swarnakar NK, Jain V, Dubey V, Mishra D, Jain NK. Enhanced oromucosal delivery of progesterone via hexosomes. Pharm Res. 2007;24(12):2223–30.
Jain S, Chauhan DS, Jain AK, Swarnakar NK, Harde H, Mahajan RR, Kumar D, Valvi PK, Das M, Datir SR, et al., inventors. Stabilization of the nanodrug delivery systems by lyophilization using universal step-wise freeze drying cycle. India patent Indian Patent Application No. 2559/DEL/2011. 2011 6 September.
Jain S, Valvi PU, Swarnakar NK, Thanki K. Gelatin coated hybrid lipid nanoparticles for oral delivery of amphotericin B. Mol Pharm. 2012;9(9):2542–53.
Rosevear FB. The microscopy of the liquid crystalline neat and middle phases of soaps and detergents. J Am Oil Chem Soc. 1954;31:628–39.
Jain S, Kumar D, Swarnakar NK, Thanki K. Polyelectrolyte stabilized multilayered liposomes for oral delivery of paclitaxel. Biomaterials. 2012;33(28):6758–68.
ICH Q1A(R2): stability testing of new drug substances and products Q1A(R2), ICH harmonized tripartite guideline; Step 4 version: February 6, 2003.
Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13(2):123–33.
Sharma M, Agrawal SK, Sharma PR, Chadha BS, Khosla MK, Saxena AK. Cytotoxic and apoptotic activity of essential oil from Ocimumviride towards COLO 205 cells. Food Chem Toxicol. 2010;48(1):336–44.
Upadhyay KK, Bhatt AN, Mishra AK, Dwarakanath BS, Jain S, Schatz C, et al. The intracellular drug delivery and anti tumor activity of doxorubicin loaded poly ([gamma]-benzyl l-glutamate)-b-hyaluronan polymersomes. Biomaterials. 2010;31(10):2882–92.
Jain V, Swarnakar NK, Mishra PR, Verma A, Kaul A, Mishra AK, et al. Paclitaxel loaded PEGylated gleceryl monooleate based nanoparticulate carriers in chemotherapy. Biomaterials. 2012;33(29):7206–20.
Putnam CD, Hammel M, Hura GL, Tainer JA. X-ray solution scattering (SAXS) combined with crystallography and computation: defining accurate macromolecular structures, conformations and assemblies in solution. Q Rev Biophys. 2007;40(3):191–285.
Dong YD, Larson I, Hanley T, Boyd BJ. Bulk and dispersed aqueous phase behavior of phytantriol: effect of vitamin E acetate and F127 polymer on liquid crystal nanostructure. Langmuir. 2006;22(23):9512–8.
Muller F, Salonen A, Glatter O. Phase behavior of Phytantriol/water bicontinuous cubic Pn3m cubosomes stabilized by Laponite disc-like particles. J Colloid Interface Sci. 2010;342(2):392–8.
Wubeante YA, Garkhal K, Neeraj K. Doxorubicin-loaded (PEG)3-PLA nanopolymersomes: effect of solvents and process parameters on formulation development and in vitro study. Mol Pharm. 2011;8:466–78.
Spicer PT, Hayden KL, Lynch ML, Ofori-Boateng A, Burns JL. Novel process for producing cubic liquid crystalline nanoparticles (cubosomes). Langmuir. 2001;17(19):5748–56.
Friberg SE, Yang H, Fei L, Sadasivan S, Rasmussen DH, Aikens PA. Preparation of vesicles from hydrotrope solutions. J Dispers Sci Technol. 1998;19(1):19–30.
Johnsson M, Lam Y, Barauskas J, Tiberg F. Aqueous phase behavior and dispersed nanoparticles of diglycerol monooleate/glycerol dioleate mixtures. Langmuir. 2005;21(11):5159–65.
Technical Bulletin, Pluronic® block copolymer NF Grades (Poloxamer NF Grades). [26 June 2013].
Jain AK, Swarnakar NK, Godugu C, Singh RP, Jain S. The effect of the oral administration of polymeric nanoparticles on the efficacy and toxicity of tamoxifen. Biomaterials. 2011;32:503–15.
Jain S, Mistry MA, Swarnakar NK. Enhanced dermal delivery of acyclovir using solid lipid nanoparticles. Drug Deliv Transl Res. 2011;1(5):395–406.
Ito T, Sun L, Bevan MA, Crooks RM. Comparison of nanoparticle size and electrophoretic mobility measurements using a carbon-nanotube-based coulter counter, dynamic light scattering, transmission electron microscopy, and phase analysis light scattering. Langmuir. 2004;20(16):6940–5.
Hyde ST. Identification of lyotropic liquid crystalline mesophases. In: Holmberg K, editor. Handbook of applied surface and colloid chemistry. J Wiley & Sons; 2001. p. 299–332.
Alexandridis P, Olsson U, Lindman B. A record nine different phases (four cubic, two hexagonal, and one lamellar lyotropic liquid crystalline and two micellar solutions) in a ternary isothermal system of an amphiphilic block copolymer and selective solvents (water and oil). Langmuir. 1998;14(10):2627–38.
Libster D, Aserin A, Wachtel E, Shoham G, Garti N. An HII liquid crystal-based delivery system for cyclosporin A: physical characterization. J Colloid Interface Sci. 2007;308(2):514–24.
Amar-Yuli I, Wachtel E, Shoshan EB, Danino D, Aserin A, Garti N. Hexosome and hexagonal phases mediated by hydration and polymeric stabilizer. Langmuir. 2007;23(7):3637–45.
Lee KW, Nguyen TH, Hanley T, Boyd BJ. Nanostructure of liquid crystalline matrix determines in vitro sustained release and in vivo oral absorption kinetics for hydrophilic model drugs. Int J Pharm. 2009;365(1–2):190–9.
Rudra A, Deepa RM, Ghosh MK, Ghosh S, Mukherjee B. Doxorubicin-loaded phosphatidylethanolamine-conjugated nanoliposomes: in vitro characterization and their accumulation in liver, kidneys, and lungs in rats. Int J Nanomedicine. 2010;5:811.
Zeng N, Gao X, Hu Q, Song Q, Xia H, Liu Z, et al. Lipid-based liquid crystalline nanoparticles as oral drug delivery vehicles for poorly water-soluble drugs: cellular interaction and in vivo absorption. Int J Nanomedicine. 2012;7:3703–18.
Muir BW, Acharya DP, Kennedy DF, Mulet X, Evans RA, Pereira SM, et al. Metal-free and MRI visible theranostic lyotropic liquid crystal nitroxide-based nanoparticles. Biomaterials. 2012;33(9):2723–33.
Ho SY, Storch J. Common mechanisms of monoacylglycerol and fatty acid uptake by human intestinal Caco-2 cells. Am J Physiol Cell Physiol. 2001;281(4):C1106–17.
Swarnakar NK, Thanki K, Jain S. Effect of co-administration of CoQ10-loaded nanoparticles on the efficacy and cardiotoxicity of doxorubicin-loaded nanoparticles. RSC Adv. 2013;3:14671–85.
Gaymalov ZZ, Yang Z, Pisarev VM, Alakhov VY, Kabanov AV. The effect of the nonionic block copolymer pluronic P85 on gene expression in mouse muscle and antigen-presenting cells. Biomaterials. 2009;30(6):1232–45.
Kabanov AV, Lemieux P, Vinogradov S, Alakhov V. Pluronic block copolymers: novel functional molecules for gene therapy. Adv Drug Deliv Rev. 2002;54(2):223–33.
Benival DM, Devarajan PV. Lipomer of doxorubicin hydrochloride for enhanced oral bioavailability. Int J Pharm. 2012;423(2):554–61.
Thomson A, Schoeller C, Keelan M, Smith L, Clandinin M. Lipid absorptions passing through the unstirred layers, brush-border membrane, and beyond. Can J Physiol Pharmacol. 1993;71(8):531–55.
Zhang Z, Ma L, Jiang S, Liu Z, Huang J, Chen L, et al. A self-assembled nanocarrier loading teniposide improves the oral delivery and drug concentration in tumor. J Control Release. 2013;166(1):30–7.
Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis. 2007;49(5):330–52.
Li K, Sung RY, Huang WZ, Yang M, Pong NH, Lee SM, et al. Thrombopoietin protects against in vitro and in vivo cardiotoxicity induced by doxorubicin. Circulation. 2006;113(18):2211–20.
Vasquez-Vivar J, Martasek P, Hogg N, Masters BS, Pritchard Jr KA, Kalyanaraman B. Endothelial nitric oxide synthase-dependent superoxide generation from adriamycin. Biochemistry. 1997;36(38):11293–7.
Singal PK, Deally CM, Weinberg LE. Subcellular effects of adriamycin in the heart: a concise review. J Mol Cell Cardiol. 1987;19(8):817–28.
Odom AL, Hatwig CA, Stanley JS, Benson AM. Biochemical determinants of Adriamycin toxicity in mouse liver, heart and intestine. Biochem Pharmacol. 1992;43(4):831–6.
Rustenbeck I, Lenzen S. Regulation of transmembrane ion transport by reaction products of phospholipase A2. II. Effects of arachidonic acid and other fatty acids on mitochondrial Ca2+ transport. Biochim Biophys Acta. 1989;982(1):147–55.
Si K, Liu J, He L, Li X, Gou W, Liu C. Caulophine protects cardiomyocytes from oxidative and ischemic injury. J Pharmacol Sci. 2010;113(4):368–77.
Acknowledgments and Disclosures
Authors are thankful to Director, NIPER for providing necessary infrastructure facilities. The work was supported by Council of Scientific and Industrial Research (CSIR), Government of India, New Delhi, India. Authors are also thankful for the technical support rendered by Mr. Rahul Mahajan.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1321 kb)
Rights and permissions
About this article
Cite this article
Swarnakar, N.K., Thanki, K. & Jain, S. Bicontinuous Cubic Liquid Crystalline Nanoparticles for Oral Delivery of Doxorubicin: Implications on Bioavailability, Therapeutic Efficacy, and Cardiotoxicity. Pharm Res 31, 1219–1238 (2014). https://doi.org/10.1007/s11095-013-1244-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-013-1244-8