Abstract
Purpose
To demonstrate the novel application of nano X-ray computed tomography (NanoXCT) for visualizing and quantifying the internal structures of pharmaceutical particles.
Methods
An Xradia NanoXCT-100, which produces ultra high-resolution and non-destructive imaging that can be reconstructed in three-dimensions (3D), was used to characterize several pharmaceutical particles. Depending on the particle size of the sample, NanoXCT was operated in Zernike Phase Contrast (ZPC) mode using either: 1) large field of view (LFOV), which has a two-dimensional (2D) spatial resolution of 172 nm; or 2) high resolution (HRES) that has a resolution of 43.7 nm. Various pharmaceutical particles with different physicochemical properties were investigated, including raw (2-hydroxypropyl)-beta-cyclodextrin (HβCD), poly (lactic-co-glycolic) acid (PLGA) microparticles, and spray-dried particles that included smooth and nanomatrix bovine serum albumin (BSA), lipid-based carriers, and mannitol.
Results
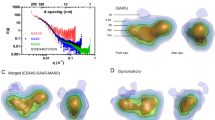
Both raw HβCD and PLGA microparticles had a network of voids, whereas spray-dried smooth BSA and mannitol generally had a single void. Lipid-based carriers and nanomatrix BSA particles resulted in low quality images due to high noise-to-signal ratio. The quantitative capabilities of NanoXCT were also demonstrated where spray-dried mannitol was found to have an average void volume of 0.117 ± 0.247 μm3 and average void-to-material percentage of 3.5%. The single PLGA particle had values of 1993 μm3 and 59.3%, respectively.
Conclusions
This study reports the first series of non-destructive 3D visualizations of inhalable pharmaceutical particles. Overall, NanoXCT presents a powerful tool to dissect and observe the interior of pharmaceutical particles, including those of a respirable size.









Similar content being viewed by others
References
Chow AHL, Tong HHY, Chattopadhyay P, Shekunov BY. Particle engineering for pulmonary drug delivery. Pharm Res. 2007;24:411–37.
Vehring R, Lechuga-Ballesteros D, Joshi V, Noga B, Dwivedi SK. Cosuspensions of microcrystals and engineered microparticles for uniform and efficient delivery of respiratory therapeutics from pressurized metered dose inhalers. Langmuir. 2012;28:15015–23.
Vanbever R, Mintzes J, Wang J, Nice J, Chen D, Batycky R, et al. Formulation and physical characterization of large porous particles for inhalation. Pharm Res. 1999;16:1735–42.
Dellamary LA, Tarara TE, Smith DJ, Woelk CH, Adractas A, Costello ML, et al. Hollow porous particles in metered dose inhalers. Pharm Res. 2000;17:168–74.
Crowder TM, Rosati JA, Schroeter JD, Hickey AJ, Martonen TB. Fundamental effects of particle morphology on lung delivery: Predictions of Stoke’s law and the particular relevance to dry powder inhaler formulation and development. Pharm Res. 2002;19:239–45.
D’Addio SM, Chan JGY, Kwok PCL, Prud’homme RK, Chan H-K. Constant size, variable density aerosol particles by ultrasonic spray freeze drying. Int J Pharm. 2012;427:185–91.
Chew NYK, Tang P, Chan H-K, Raper JA. How much particle surface corrugation is sufficient to improve aerosol performance of powders? Pharm Res. 22:148–152 (2005).
Heng D, Tang P, Cairney J, Chan H-K, Cutler D, Salama R, et al. Focused-ion-beam milling: A novel approach to probing the interior of particles used for inhalation aerosols. Pharm Res. 2007;24:1608–17.
Chew NYK, Chan H-K. Influence of particle size, air flow, and inhaler device on the dispersion of mannitol powders as aerosols. Pharm Res. 1999;16:1098–103.
Chew NYK, Chan H-K. Use of solid corrugated particles to enhance powder aerosol performance. Pharm Res. 2001;18:1570–7.
Chiou H, Chan H-K, Heng D, Prud’homme RK, Raper JA. A novel production method for inhalable cyclosporine A powders by confined liquid impinging jet precipitation. J Aerosol Sci. 2008;39:500–509.
Zhu B, Traini D, Chan H-K, Young PM. The effect of ethanol on the formation and physico-chemical properties of particles generated from budesonide solution-based pressurized metered-dose inhalers. Drug Dev Ind Pharm. 2013;39:1625–37.
Larson D, Foord D, Petford-Long A, Anthony T, Rozdilsky I, Cerezo A, et al. Focused ion-beam milling for field-ion specimen preparation: Preliminary investigations. Ultramicroscopy. 1998;75:147–59.
Miller MK, Russell KF, Thompson K, Alvis R, Larson DJ. Review of atom probe FIB-based specimen preparation methods. Microsc Microanal. 2007;13:428–36.
Vieu C, Gierak J, Launois H, Aign T, Meyer P, Jamet J, et al. High resolution magnetic patterning using focused ion beam irradiation. Microelectron Eng. 2000;53:191–4.
Fu Y, Bryan NKA, Shing O, Hung N. Influence of the redeposition effect for focused ion beam 3D micromachining in silicon. Int J Adv Manuf Technol. 2000;16:877–80.
Shurand J, Price R. Advanced microscopy techniques to assess solid-state properties of inhalation medicines. Adv Drug Deliv Rev. 2012;64:369–82.
Ketchamand RA, Carlson WD. Acquisition, optimization and interpretation of X-ray computed tomographic imagery: Applications to the geosciences. Comput Geosci. 2001;27:381–400.
Taina I, Heck R, Elliot T. Application of X-ray computed tomography to soil science: A literature review. Can J Soil Sci. 2008;88:1–19.
Momose A, Takeda T, Itai Y, Hirano K. Phase–contrast X–ray computed tomography for observing biological soft tissues. Nat Med. 1996;2:473–5.
Farber L, Tardos G, Michaels JN. Use of X-ray tomography to study the porosity and morphology of granules. Powder Technol. 2003;132:57–63.
Sinka IC, Burch SF, Tweed JH, Cunningham JC. Measurement of density variations in tablets using X-ray computed tomography. Int J Pharm. 2004;271:215–24.
Fu X, Dutt M, Bentham AC, Hancock BC, Cameron RE, Elliott JA. Investigation of particle packing in model pharmaceutical powders using X-ray microtomography and discrete element method. Powder Technol. 2006;167:134–40.
Attwood D. Microscopy: Nanotomography comes of age. Nature. 2006;442:642–3.
Sakdinawatand A, Attwood D. Nanoscale X-ray imaging Nature Photonics. 2010;4:840–8.
Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470:543–7.
Adi S, Adi H, Tang P, Traini D, Chan H-K, Young PM. Micro-particle corrugation, adhesion and inhalation aerosol efficiency. Eur J Pharm Sci. 2008;35:12–8.
Kwok P, Tunsirikongkon A, Glover W, Chan H-K. Formation of protein nano-matrix particles with controlled surface architecture for respiratory drug delivery. Pharm Res. 2011;28:788–96.
Tarara TE, Hartman MS, Gill H, Kennedy AA, Weers JG. Characterization of suspension-based metered dose inhaler formulations composed of spray-dried budesonide microcrystals dispersed in HFA-134a. Pharm Res. 2004;21:1607–14.
Weers JG, Tarara TE, Gill H, English BS, Dellamary LA. Homodispersion technology for HFA suspensions: particle engineering to reduce dosing variance. Respiratory Drug Delivery. 2000;2:91–7.
Iveyand JW, Vehring R. The use of modeling in spray drying of emulsions and suspensions accelerates formulation and process development. Comput Chem Eng. 2010;34:1036–40.
D’Sa D, Chan H-K, Chrzanowski W. Attachment of micro- and nano-particles on tipless cantilevers for colloidal probe microscopy. J Colloid Interface Sci (2014).
Russ JC. The Image Processing Handbook. London: Boca Raton; 1995.
Elverssonand J, Millqvist-Fureby A. Particle size and density in spray drying — Effects of carbohydrate properties. J Pharm Sci. 2005;94:2049–60.
Diaz A, Trtik P, Guizar-Sicairos M, Menzel A, Thibault P, Bunk O. Quantitative X-ray phase nanotomography. Phys Rev B. 2012;85:020104.
Brisard S, Chae RS, Bihannic I, Michot L, Guttmann P, Thieme J, et al. Morphological quantification of hierarchical geomaterials by X-ray nano-CT bridges the gap from nano to micro length scales. Am Mineral. 2012;97:480–3.
Dong P, Pacureanu A, Zuluaga MA, Olivier C, Frouin F, Grimal Q, Peyrin F. A new quantitative approach for estimating bone cell connections from nano-CT images, Engineering in Medicine and Biology Society (EMBC), 2013 35th Annual International Conference of the IEEE, 2013, pp. 3694–3697.
Trtik P, Soos M, Münch B, Lamprou A, Mokso R, Stampanoni M. Quantification of a single aggregate inner porosity and pore accessibility using hard X-ray phase-contrast nanotomography. Langmuir. 2011;27:12788–91.
Kerckhofs G, Sainz J, Wevers M, Van de Putte T, Schrooten J. Contrast-enhanced nanofocus computed tomography images the cartilage subtissue architecture in three dimensions. Eur Cell Mater. 2013;25:179–89.
D’Sa D, Chan H-K, Chrzanowski W. Predicting physical stability in pressurized metered dose inhalers via dwell and instantaneous force colloidal probe microscopy. Eur J Pharm Sci (Submitted).
ACKNOWLEDGMENTS AND DISCLOSURES
Jennifer Wong and Dexter D'Sa contributed equally to this work.
The authors acknowledge the facilities and technical assistance of Steve Moody (SEM and FIB Specialist) and the Australian Microscopy & Microanalysis Research Facility at the Australian Centre for Microscopy & Microanalysis, University of Sydney. Jennifer Wong, Dexter D’Sa and John Chan were recipients of the Australian Postgraduate Award, and Dexter D’Sa was also the recipient of the Australian IPRS scholarship. This work was supported under the Australian Research Council“s Discovery Projects funding scheme (projects DP120102778 & 110105161).
Author information
Authors and Affiliations
Corresponding author
SUPPLEMENTARY MATERIAL
SUPPLEMENTARY MATERIAL
A video showing the reconstructed 3D particles of all samples rotating 360° around an axis can be found in the supplementary materials.
Rights and permissions
About this article
Cite this article
Wong, J., D’Sa, D., Foley, M. et al. NanoXCT: A Novel Technique to Probe the Internal Architecture of Pharmaceutical Particles. Pharm Res 31, 3085–3094 (2014). https://doi.org/10.1007/s11095-014-1401-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-014-1401-8