Abstract
Purpose
The goals of this study were to determine: 1) the impact of surfactants on the “amorphous solubility”; 2) the thermodynamic supersaturation in the presence of surfactant micelles; 3) the mechanism of solute solubilization by surfactant micelles in supersaturated solutions.
Methods
The crystalline and amorphous solubility of atazanavir was determined in the presence of varying concentrations of micellar sodium dodecyl sulfate (SDS). Flux measurements, using a side-by-side diffusion cell, were employed to determine the free and micellar-bound drug concentrations. The solubilization mechanism as a function of atazanavir concentration was probed using fluorescence spectroscopy. Pulsed gradient spin-echo proton nuclear magnetic resonance (PGSE-NMR) spectroscopy was used to determine the change in micelle size with a change in drug concentration.
Results
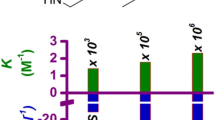
Changes in the micelle/water partition coefficient, K m/w , as a function of atazanavir concentration led to erroneous estimates of the supersaturation when using concentration ratios. In contrast, determining the free drug concentration using flux measurements enabled improved determination of the thermodynamic supersaturation in the presence of micelles. Fluorescence spectroscopic studies suggested that K m/w changed based on the location of atazanavir solubilization which in turn changed with concentration. Thus, at a concentration equivalent to the crystalline solubility, atazanavir is solubilized by adsorption at the micelle corona, whereas in highly supersaturated solutions it is also solubilized in the micellar core. This difference in solubilization mechanism can lead to a breakdown in the prediction of amorphous solubility in the presence of SDS as well as challenges with determining supersaturation. PGSE-NMR suggested that the size of the SDS micelle is not impacted at the crystalline solubility of the drug but increases when the drug concentration reaches the amorphous solubility, in agreement with the proposed changes in solubilization mechanism.
Conclusions
Micellar solubilization of atazanavir is complex, with the solubilization mechanism changing with differences in the degree of (super)saturation. This can result in erroneous predictions of the amorphous solubility and thermodynamic supersaturation in the presence of solubilizing additives. This in turn hinders understanding of the driving force for phase transformations and membrane transport, which is essential to better understand supersaturating dosage forms.













Similar content being viewed by others
Abbreviations
- ASD:
-
Amorphous solid dispersion
- ATZ:
-
Atazanavir
- CMC:
-
Critical micelle concentration
- FRET:
-
Förster resonance energy transfer
- GLPS:
-
Glass liquid phase separation
- HPLC:
-
High performance liquid chromatography
- LLPS:
-
Liquid liquid phase separation
- PGSE-NMR:
-
Pulsed gradient spin-echo proton nuclear magnetic resonance
- SDS:
-
Sodium dodecyl sulfate
References
Hogan JC. Combinatorial chemistry in drug discovery. Nat Biotechnol. 1997;15(4):328–30.
Broach JR, Thorner J. High-throughput screening for drug discovery. Nature. 1996;384(6604 Suppl):14–6.
Carnero A. High throughput screening in drug discovery. Clin Transl Oncol. 2006;8(7):482–90.
Lipinski CA. Drug-like properties and the causes of poor solubility and poor permeability. J Pharmacol Toxicol Methods. 2000;44(1):235–49.
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 1997;23(1–3):3–25.
Ran Y, Zhao L, Xu Q, Yalkowsky SH. Solubilization of cyclosporin a. AAPS PharmSciTech. 2001;2(1):23–6.
Loftsson T, Magnúsdóttir A, Másson M, Sigurjónsdóttir JF. Self-association and cyclodextrin solubilization of drugs. J Pharm Sci. 2002;91(11):2307–16.
Müller BW, Brauns U. Solubilization of drugs by modified β-cyclodextrins. Int J Pharm. 1985;26(1):77–88.
Neslihan Gursoy R, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother. 2004;58(3):173–82.
Anby MU, Williams HD, McIntosh M, Benameur H, Edwards GA, Pouton CW, Porter CJH. Lipid digestion as a trigger for supersaturation: evaluation of the impact of supersaturation stabilization on the in vitro and in vivo performance of self-emulsifying drug delivery systems. Mol Pharm. 2012;9(7):2063–79.
Williams HD, Trevaskis NL, Yeap YY, Anby MU, Pouton CW, Porter CJ. Lipid-based formulations and drug supersaturation: harnessing the unique benefits of the lipid digestion/absorption pathway. Pharm Res. 2013;30(12):2976–92.
Serajuddin ATM. Salt formation to improve drug solubility. Adv Drug Deliv Rev. 2007;59(7):603–16.
Thakuria R, Delori A, Jones W, Lipert MP, Roy L, Rodríguez-Hornedo N. Pharmaceutical cocrystals and poorly soluble drugs. Int J Pharm. 2013;453(1):101–25.
Newman A, Knipp G, Zografi G. Assessing the performance of amorphous solid dispersions. J Pharm Sci. 2012;101(4):1355–77.
Brouwers J, Tack J, Augustijns P. In vitro behavior of a phosphate ester prodrug of amprenavir in human intestinal fluids and in the Caco-2 system: illustration of intraluminal supersaturation. Int J Pharm. 2007;336(2):302–9.
Kapoor M, Siegel RA. Prodrug/enzyme based acceleration of absorption of hydrophobic drugs: an in vitro study. Mol Pharm. 2013;10(9):3519–24.
Miller JM, Beig A, Krieg BJ, Carr RA, Borchardt TB, Amidon GE, Amidon GL, Dahan A. The solubility–permeability interplay: mechanistic modeling and predictive application of the impact of micellar Solubilization on intestinal permeation. Mol Pharm. 2011;8(5):1848–56.
Miller JM, Beig A, Carr RA, Spence JK, Dahan A. A win–win solution in oral delivery of lipophilic drugs: supersaturation via amorphous solid dispersions increases apparent solubility without sacrifice of intestinal membrane permeability. Mol Pharm. 2012;9(7):2009–16.
Dahan A, Miller JM, Hoffman A, Amidon GE, Amidon GL. The solubility–permeability interplay in using cyclodextrins as pharmaceutical solubilizers: mechanistic modeling and application to progesterone. J Pharm Sci. 2010;99(6):2739–49.
Miller JM, Beig A, Carr RA, Webster GK, Dahan A. The solubility–permeability interplay when using Cosolvents for Solubilization: revising the way we use solubility-enabling formulations. Mol Pharm. 2012;9(3):581–90.
Hou H, Siegel RA. Enhanced permeation of diazepam through artificial membranes from supersaturated solutions. J Pharm Sci. 2006;95(4):896–905.
Leveque N, Raghavan SL, Lane ME, Hadgraft J. Use of a molecular form technique for the penetration of supersaturated solutions of salicylic acid across silicone membranes and human skin in vitro. Int J Pharm. 2006;318(1–2):49–54.
Megrab NA, Williams AC, Barry BW. Oestradiol permeation through human skin and silastic membrane: effects of propylene glycol and supersaturation. J Control Release. 1995;36(3):277–94.
Pellett MA, Castellano S, Hadgraft J, Davis AF. The penetration of supersaturated solutions of piroxicam across silicone membranes and human skin in vitro. J Control Release. 1997;46(3):205–14.
Mullin JW. Solutions and solubility. In: Crystallization. Oxford: Butterworth-Heinemann; 2001. p. 86–134.
Higuchi T. Physical chemical analysis of percutaneous absorption process from creams and ointments. J Soc Cosmet Chem. 1960;11(2):85–97.
Twist J, Zatz J. Characterization of solvent-enhanced permeation through a skin model membrane. J Soc Cosmet Chem. 1988;39(5):324–4.
Sigurðoardóttir AM, Loftsson T. The effect of polyvinylpyrrolidone on cyclodextrin complexation of hydrocortisone and its diffusion through hairless mouse skin. Int J Pharm. 1995;126(1–2):73–8.
Loftsson T, Sigurðardóttir AM. The effect of polyvinylpyrrolidone and hydroxypropyl methylcellulose on HPβCD complexation of hydrocortisone and its permeability through hairless mouse skin. Eur J Pharm Sci. 1994;2(4):297–301.
Kim J-H, Choi H-K. Effect of additives on the crystallization and the permeation of ketoprofen from adhesive matrix. Int J Pharm. 2002;236(1–2):81–5.
Ilevbare GA, Taylor LS. Liquid–liquid phase separation in highly supersaturated aqueous solutions of poorly water-soluble drugs: implications for solubility enhancing formulations. Cryst Growth Des. 2013;13(4):1497–509.
Mosquera-Giraldo LI, Taylor LS. Glass–liquid phase separation in highly supersaturated aqueous solutions of Telaprevir. Mol Pharm. 2015;12(2):496–503.
Indulkar AS, Box KJ, Taylor R, Ruiz R, Taylor LS. pH-dependent liquid–liquid phase separation of highly supersaturated solutions of weakly basic drugs. Mol Pharm. 2015;12(7):2365–77.
Jackson MJ, Kestur US, Hussain MA, Taylor LS. Characterization of supersaturated Danazol solutions–impact of polymers on solution properties and phase transitions. Pharm Res. 2016;33(5):1–13.
Raina SA, Zhang GGZ, Alonzo DE, Wu J, Zhu D, Catron ND, Gao Y, Taylor LS. Enhancements and limits in drug membrane transport using supersaturated solutions of poorly water soluble drugs. J Pharm Sci. 103(9):2736–48.
Indulkar AS, Gao Y, Raina SA, Zhang GGZ, Taylor LS. Exploiting the phenomenon of liquid–liquid phase separation for enhanced and sustained membrane transport of a poorly water-soluble drug. Mol Pharm. 2016;13(6):2059–69.
Taylor LS, Zhang GGZ. Physical chemistry of supersaturated solutions and implications for oral absorption. Adv Drug Deliv Rev. 2016;101:122–42.
Jantratid E, Dressman J. Biorelevant dissolution media simulating the proximal human gastrointestinal tract: an update. Dissolution Technologies. 2009;16(3):21–5.
Dressman JB, Amidon GL, Reppas C, Shah VP. Dissolution testing as a prognostic tool for oral drug absorption: immediate release dosage forms. Pharm Res. 1998;15(1):11–22.
Riethorst D, Mols R, Duchateau G, Tack J, Brouwers J, Augustijns P. Characterization of human duodenal fluids in fasted and fed state conditions. J Pharm Sci. 2016;105(2):673–81.
Mazer NA, Benedek GB, Carey MC. Quasielastic light-scattering studies of aqueous biliary lipid systems. Mixed micelle formation in bile salt-lecithin solutions. Biochemistry. 1980;19(4):601–15.
Small DM, Penkett SA, Chapman D. Studies on simple and mixed bile salt micelles by nuclear magnetic resonance spectroscopy. Biochimica et Biophysica Acta, Lipids and Lipid Metabolism. 1969;176(1):178–89.
Hammad MA, Müller BW. Increasing drug solubility by means of bile salt–phosphatidylcholine-based mixed micelles. Eur J Pharm Biopharm. 1998;46(3):361–7.
Rosoff M, Serajuddin ATM. Solubilization of diazepam in bile salts and in sodium cholate-lecithin-water phases. Int J Pharm. 1980;6(2):137–46.
Raina SA, Zhang GG, Alonzo DE, Wu J, Zhu D, Catron ND, Gao Y, Taylor LS. Impact of solubilizing additives on supersaturation and membrane transport of drugs. Pharm Res. 2015;32(10):3350–64.
Feng S. Studies on drug solubilization mechanism in simple micelle systems. Dissertation, Lexington: University of Kentucky; 2009.
Rosen MJ, Kunjappu JT. Solubilization by solutions of surfactants: micellar catalysis. In: Surfactants and interfacial phenomena. Hoboken: Wiley; 2012. p. 202–34.
Eriksson J, Gillberg G. NMR-studies of the solubilization of aromatic compounds in cetyltrimethylammonium bromide solution II. Acta Chem Scand. 1966;20(8):2019–27.
Jobe DJ, Reinsborough VC, Wetmore SD. Sodium dodecyl sulfate micellar aggregation numbers in the presence of cyclodextrins. Langmuir. 1995;11(7):2476–9.
Hansson P, Jönsson B, Ström C, Söderman O. Determination of micellar aggregation numbers in dilute surfactant systems with the fluorescence quenching method. J Phys Chem B. 2000;104(15):3496–506.
Alargova R, Kochijashky I, Sierra M, Zana R. Micelle aggregation numbers of surfactants in aqueous solutions: a comparison between the results from steady-state and time-resolved fluorescence quenching. Langmuir. 1998;14(19):5412–8.
Malliaris A. Static fluorescence quenching in the study of micellar systems. In: Hoffmann H, editor. New trends in Colloid Science. New York City: Springer; 1987. p. 161–6.
Infelta PP. Fluorescence quenching in micellar solutions and its application to the determination of aggregation numbers. Chem Phys Lett. 1979;61(1):88–91.
Turro NJ, Yekta A. Luminescent probes for detergent solutions. A simple procedure for determination of the mean aggregation number of micelles. J Am Chem Soc. 1978;100(18):5951–2.
Hoffman JD. Thermodynamic driving force in nucleation and Growth processes. J Chem Phys. 1958;29(5):1192–3.
Murdande SB, Pikal MJ, Shanker RM, Bogner RH. Solubility advantage of amorphous pharmaceuticals: I. A thermodynamic analysis. J Pharm Sci. 2010;99(3):1254–64.
Murdande SB, Pikal MJ, Shanker RM, Bogner RH. Solubility advantage of amorphous pharmaceuticals: II. Application of quantitative thermodynamic relationships for prediction of solubility enhancement in structurally diverse insoluble pharmaceuticals. Pharm Res. 2010;27(12):2704–14.
Purohit HS, Taylor LS. Phase separation kinetics in amorphous solid dispersions upon exposure to water. Mol Pharm. 2015;12(5):1623–35.
Fuguet E, Ràfols C, Rosés M, Bosch E. Critical micelle concentration of surfactants in aqueous buffered and unbuffered systems. Anal Chim Acta. 2005;548(1–2):95–100.
Corrin ML. The effect of salts and chain length on the critical concentrations of colloidal electrolytes. J Colloid Sci. 1948;3(4):333–8.
Tamori K, Watanabe Y, Esumi K. The partitioning of pyrene-3-carboxaldehyde at the micelle/bulk interface estimated by the fluorescence method. Langmuir. 1992;8(9):2344–6.
Phillips J. The energetics of micelle formation. Trans Faraday Soc. 1955;51:561–9.
Quina FH, Nassar PM, Bonilha JBS, Bales BL. Growth of sodium dodecyl sulfate micelles with detergent concentration. J Phys Chem. 1995;99(46):17028–31.
Florence AT, Attwood D. Surfactants. In: Physicochemical principles of pharmacy. London: Macmillan Education UK; 1998. p. 199–251.
Lee BH, Christian SD, Tucker EE, Scamehorn JF. Solubilization of mono-and dichlorophenols by hexadecylpyridinium chloride micelles. Effects of substituent groups. Langmuir. 1990;6(1):230–5.
Choucair A, Eisenberg A. Interfacial Solubilization of model amphiphilic molecules in block copolymer micelles. J Am Chem Soc. 2003;125(39):11993–2000.
Croy SR, Kwon GS. Polysorbate 80 and Cremophor EL micelles deaggregate and solubilize nystatin at the core–corona interface. J Pharm Sci. 2005;94(11):2345–54.
Adhikary R, Carlson PJ, Kee TW, Petrich JW. Excited-state intramolecular hydrogen atom transfer of curcumin in surfactant micelles. J Phys Chem B. 2010;114(8):2997–3004.
Moroi Y, Mitsunobu K, Morisue T, Kadobayashi Y, Sakai M. Solubilization of benzene, naphthalene, anthracene, and pyrene in 1-Dodecanesulfonic acid micelle. J Phys Chem. 1995;99(8):2372–6.
Gadelle F, Koros WJ, Schechter RS. Solubilization isotherms of aromatic solutes in surfactant aggregates. J Colloid Interface Sci. 1995;170(1):57–64.
Powell JJ, Faria N, Thomas-McKay E, Pele LC. Origin and fate of dietary nanoparticles and microparticles in the gastrointestinal tract. J Autoimmun. 2010;34(3):J226–33.
Jani P, Halbert GW, Langridge J, Florence AT. Nanoparticle uptake by the rat gastrointestinal mucosa: quantitation and particle size dependency. J Pharm Pharmacol. 1990;42(12):821–6.
Mukerjee P, Cardinal JR. Benzene derivatives and naphthalene solubilized in micelles. Polarity of microenvironments, location and distribution in micelles, and correlation with surface activity in hydrocarbon-water systems. J Phys Chem. 1978;82(14):1620–7.
Smith GA, Christian SD, Tucker EE, Scamehorn JF. Solubilization of hydrocarbons by surfactant micelles and mixed micelles. J Colloid Interface Sci. 1989;130(1):254–65.
Lu J, Ormes JD, Lowinger M, Xu W, Mitra A, Mann AK, Litster JD, Taylor LS. Impact of endogenous bile salts on the thermodynamics of supersaturated active pharmaceutical ingredient solutions. Cryst Growth Des. 2017;17(3):1264–75.
Alonzo DE, Gao Y, Zhou D, Mo H, Zhang GGZ, Taylor LS. Dissolution and precipitation behavior of amorphous solid dispersions. J Pharm Sci. 2011;100(8):3316–31.
Jackson MJ, Kestur US, Hussain MA, Taylor LS. Dissolution of Danazol amorphous solid dispersions: supersaturation and phase behavior as a function of drug loading and polymer type. Mol Pharm. 2016;13(1):223–31.
Almeida e Sousa L, Reutzel-Edens SM, Stephenson GA, Taylor LS. Supersaturation potential of salt, co-crystal, and amorphous forms of a model Weak Base. Cryst Growth Des. 2016;16(2):737–48.
Carlert S, Pålsson A, Hanisch G, Von Corswant C, Nilsson C, Lindfors L, Lennernäs H, Abrahamsson B. Predicting intestinal precipitation—a case example for a basic BCS class II drug. Pharm Res. 2010;27(10):2119–30.
Wohnsland F, Faller B. High-throughput permeability pH profile and high-throughput alkane/water log P with artificial membranes. J Med Chem. 2001;44(6):923–30.
Liu H, Sabus C, Carter GT, Du C, Avdeef A, Tischler M. In vitro permeability of poorly aqueous soluble compounds using different Solubilizers in the PAMPA assay with liquid chromatography/mass spectrometry detection. Pharm Res. 2003;20(11):1820–6.
Borchardt RT, Hidalgo I, Raub T, Borchardt R. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterol. 1989;96(3):736–49.
Artursson P, Karlsson J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem Biophys Res Commun. 1991;175(3):880–5.
Hubatsch I, Ragnarsson EGE, Artursson P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat Protoc. 2007;2(9):2111–9.
Poelma FGJ, Breäs R, Tukker JJ, Crommelin DJA. Intestinal absorption of drugs. The influence of mixed micelles on on the disappearance kinetics of drugs from the small intestine of the rat. J Pharm Pharmacol. 1991;43(5):317–24.
Katneni K, Charman SA, Porter CJH. Permeability assessment of poorly water-soluble compounds under solubilizing conditions: the reciprocal permeability approach. J Pharm Sci. 2006;95(10):2170–85.
Acknowledgments and Disclosures
We would like to acknowledge AbbVie Inc. for providing research funding for this project. Purdue University and AbbVie jointly participated in study design, research, data collection, analysis and interpretation of data, writing, reviewing, and approving the publication. Anura S. Indulkar is a graduate student at Purdue University; Lynne S. Taylor is a professor at Purdue University; Huaping Mo is an Associate Director of Purdue Interdepartmental NMR Facility at Purdue University. They all have no additional conflicts of interest to report. Shweta A. Raina, Yi Gao, and Geoff G. Z. Zhang are employees of AbbVie and may own AbbVie stock.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Indulkar, A.S., Mo, H., Gao, Y. et al. Impact of Micellar Surfactant on Supersaturation and Insight into Solubilization Mechanisms in Supersaturated Solutions of Atazanavir. Pharm Res 34, 1276–1295 (2017). https://doi.org/10.1007/s11095-017-2144-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-017-2144-0