Abstract
Purpose
Inhalation therapy is popular to treat lower respiratory tract infections. Azithromycin is effective against some bacteria that cause respiratory tract infections; but it has poor water solubility that may limit its efficacy when administrated as inhalation therapy. In this study, dry powder inhaler formulations were developed by co-spray drying azithromycin with L-leucine with a purpose to improve dissolution.
Methods
The produced powder formulations were characterized regarding particle size, morphology, surface composition and in-vitro aerosolization performance. Effects of L-leucine on the solubility and in-vitro dissolution of azithromycin were also evaluated.
Results
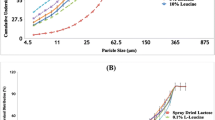
The spray dried azithromycin alone formulation exhibited a satisfactory aerosol performance with a fine particle fraction (FPF) of 62.5 ± 4.1%. Addition of L-leucine in the formulation resulted in no significant change in particle morphology and FPF, which can be attributed to enrichment of azithromycin on the surfaces of composite particles. Importantly, compared with the spray-dried amorphous azithromycin alone powder, the co-spray dried powder formulations of azithromycin and L-leucine demonstrated a substantially enhanced in-vitro dissolution rate. Such enhanced dissolution of azithromycin could be attributed to the formation of composite system and the acidic microenvironment around azithromycin molecules created by the dissolution of acidic L-leucine in the co-spray dried powder. Fourier transform infrared spectroscopic data showed intermolecular interactions between azithromycin and L-leucine in the co-spray dried formulations.
Conclusions
We developed the dry powder formulations with satisfactory aerosol performance and enhanced dissolution for a poorly water soluble weak base, azithromycin, by co-spray drying with an amino acid, L-leucine.









Similar content being viewed by others
References
Wagner T, Soong G, Sokol S, Saiman L, Prince A. Effects of azithromycin on clinical isolates of pseudomonas aeruginosa from cystic fibrosis patients. Chest. 2005;128(2):912–9.
Southern KW, Barker PM. Azithromycin for cystic fibrosis. Eur Respir J. 2004;24(5):834–8.
Wilms EB, Touw DJ, Heijerman HGM, van der Ent CK. Azithromycin maintenance therapy in patients with cystic fibrosis: a dose advice based on a review of pharmacokinetics, efficacy, and side effects. Pediatr Pulmonol. 2012;47(7):658–65.
Amsden GW. Anti-inflammatory effects of macrolides--an underappreciated benefit in the treatment of community-acquired respiratory tract infections and chronic inflammatory pulmonary conditions? J Antimicrob Chemother. 2005;55(1):10–21.
Gotfried MH. Macrolides for the treatment of chronic sinusitis, asthma, and COPD. Chest. 2004;125(2, Supplement):52S–61S.
Favre-Bonté S, Köhler T, Van Delden C. Biofilm formation by Pseudomonas aeruginosa: role of the C4-HSL cell-to-cell signal and inhibition by azithromycin. J Antimicrob Chemother. 2003;52(4):598–604.
Han MK, Tayob N, Murray S, Dransfield MT, Washko G, Scanlon PD, et al. Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy. Am J Respir Crit Care Med. 2014;189(12):1503–8.
Catherinot E, Roux AL, Vibet MA, Bellis G, Lemonnier L, Le Roux E, et al. Inhaled therapies, azithromycin and Mycobacterium abscessus in cystic fibrosis patients. Eur Respir J. 2013;41(5):1101–6.
Chang AB, Grimwood K, White AV, Maclennan C, Sloots TP, Sive A, et al. Randomized placebo-controlled trial on azithromycin to reduce the morbidity of bronchiolitis in Indigenous Australian infants: rationale and protocol. Trials. 2011;12:94.
Ekici A, Ekici M, Erdemoglu AK. Effect of azithromycin on the severity of bronchial hyperresponsiveness in patients with mild asthma. J Asthma Off J Assoc Care Asthma. 2002;39(2):181–5.
Hopkins S. Clinical toleration and safety of azithromycin. Am J Med. 1991;91(3):S40–5.
Wallace MR, Miller LK, Nguyen MT, Shields AR. Ototoxicity with azithromycin. Lancet (Lond Engl). 1994;343(8891):241.
Hickey AJ, Lu D, Ashley ED, Stout J. Inhaled azithromycin therapy. J Aerosol Med. 2006;19(1):54–60.
Frijlink H, De Boer A. Dry powder inhalers for pulmonary drug delivery. Expert Opin Drug Deliv. 2004;1(1):67–86.
Hickey A, Durham P, Dharmadhikari A, Nardell E. Inhaled drug treatment for tuberculosis: past progress and future prospects. J Control Release. 2016;240:127–34.
Velkov T, Abdul Rahim N, Zhou Q, Chan H-K, Li J. Inhaled anti-infective chemotherapy for respiratory tract infections: successes, challenges and the road ahead. Adv Drug Deliv Rev. 2015;85:65–82.
Zhou QT, Tang P, Leung SSY, Chan JGY, Chan H-K. Emerging inhalation aerosol devices and strategies: where are we headed? Adv Drug Deliv Rev. 2014;75:3–17.
Timsina MP, Martin GP, Marriott C, Ganderton D, Yianneskis M. Drug-delivery to the respiratory-tract using dry powder inhalers. Int J Pharm. 1994;101(1–2):1–13.
Weers J. Inhaled antimicrobial therapy–barriers to effective treatment. Adv Drug Deliv Rev. 2015;85:24–43.
Buttini F, Colombo P, Rossi A, Sonvico F, Colombo G. Particles and powders: tools of innovation for non-invasive drug administration. J Control Release. 2012;161(2):693–702.
Chan HK, Chew NYK. Novel alternative methods for the delivery of drugs for the treatment of asthma. Adv Drug Deliv Rev. 2003;55(7):793–805.
de Boer AH, Chan HK, Price R. A critical view on lactose-based drug formulation and device studies for dry powder inhalation: which are relevant and what interactions to expect? Adv Drug Deliv Rev. 2012;64(3):257–74.
Smyth HD, Hickey AJ. Carriers in drug powder delivery. Am J Drug Deliv. 2005;3(2):117–32.
Zhou QT, Leung SS, Tang P, Parumasivam T, Loh ZH, Chan HK. Inhaled formulations and pulmonary drug delivery systems for respiratory infections. Adv Drug Deliv Rev. 2015;85:83–99.
Lin Y-W, Wong J, Qu L, Chan H-K, Zhou QT. Powder production and particle engineering for dry powder inhaler formulations. Curr Pharm Des. 2015;21(27):3902–16.
Thakkar SG, Fathe K, Smyth HD. Amorphous or crystalline? A comparison of particle engineering methods and selection. Curr Pharm Des. 2015;21(40):5789–801.
Cun D, Wan F, Yang M. Formulation strategies and particle engineering technologies for pulmonary delivery of biopharmaceuticals. Curr Pharm Des. 2015;21(19):2599–610.
Vehring R. Pharmaceutical particle engineering via spray drying. Pharm Res. 2008;25(5):999–1022.
Bohr AP, Boetker J, Rades T, Rantanen J, Yang M. Application of spray-drying and electrospraying/electospinning for poorly watersoluble drugs: a particle engineering approach. Curr Pharm Des. 2014;20(3):325–48.
Young PM, Salama RO, Zhu B, Phillips G, Crapper J, Chan HK, et al. Multi-breath dry powder inhaler for delivery of cohesive powders in the treatment of bronchiectasis. Drug Dev Ind Pharm. 2015;41(5):859–65.
Li X, Vogt FG, Hayes D, Mansour HM. Design, characterization, and aerosol dispersion performance modeling of advanced co-spray dried antibiotics with mannitol as respirable microparticles/nanoparticles for targeted pulmonary delivery as dry powder inhalers. J Pharm Sci. 2014;103(9):2937–49.
Li X, Vogt FG, Hayes D Jr, Mansour HM. Physicochemical characterization and aerosol dispersion performance of organic solution advanced spray-dried microparticulate/nanoparticulate antibiotic dry powders of tobramycin and azithromycin for pulmonary inhalation aerosol delivery. Eur J Pharm Sci. 2014;52:191–205.
Zhang Y, Wang X, Lin X, Liu X, Tian B, Tang X. High azithromycin loading powders for inhalation and their in vivo evaluation in rats. Int J Pharm. 2010;395(1–2):205–14.
Highlights of prescribing information for ZITHROMAX. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/050710s043,050711s040,050784s027lbl.pdf. Accessed 10 Dec 2017.
Loira-Pastoriza C, Todoroff J, Vanbever R. Delivery strategies for sustained drug release in the lungs. Adv Drug Deliv Rev. 2014;75:81–91.
Singh A, Van den Mooter G. Spray drying formulation of amorphous solid dispersions. Adv Drug Deliv Rev. 2016;100:27–50.
Chen L, Okuda T, Lu X-Y, Chan H-K. Amorphous powders for inhalation drug delivery. Adv Drug Deliv Rev. 2016;100(Supplement C):102–15.
Laitinen R, Lobmann K, Grohganz H, Strachan C, Rades T. Amino acids as co-amorphous excipients for simvastatin and glibenclamide: physical properties and stability. Mol Pharm. 2014;11(7):2381–9.
Chavan RB, Thipparaboina R, Kumar D, Shastri NR. Co amorphous systems: a product development perspective. Int J Pharm. 2016;515(1–2):403–15.
Lenz E, Jensen KT, Blaabjerg LI, Knop K, Grohganz H, Löbmann K, et al. Solid-state properties and dissolution behaviour of tablets containing co-amorphous indomethacin–arginine. Eur J Pharm Biopharm. 2015;96:44–52.
Jensen KT, Larsen FH, Cornett C, Löbmann K, Grohganz H, Rades T. Formation mechanism of coamorphous drug-amino acid mixtures. Mol Pharm. 2015;12(7):2484–92.
Löbmann K, Laitinen R, Strachan C, Rades T, Grohganz H. Amino acids as co-amorphous stabilizers for poorly water-soluble drugs – part 2: molecular interactions. Eur J Pharm Biopharm. 2013;85(3, Part B):882–8.
Aucamp M, Odendaal R, Liebenberg W, Hamman J. Amorphous azithromycin with improved aqueous solubility and intestinal membrane permeability. Drug Dev Ind Pharm. 2015;41(7):1100–8.
Arora S, Haghi M, Young PM, Kappl M, Traini D, Jain S. Highly respirable dry powder inhalable formulation of voriconazole with enhanced pulmonary bioavailability. Expert Opin Drug Deliv. 2016;13(2):183–93.
Rabbani NR, Seville PC. The influence of formulation components on the aerosolisation properties of spray-dried powders. J Control Release. 2005;110(1):130–40.
Chew NY, Shekunov BY, Tong HH, Chow AH, Savage C, Wu J, et al. Effect of amino acids on the dispersion of disodium cromoglycate powders. J Pharm Sci. 2005;94(10):2289–300.
Li L, Sun S, Parumasivam T, Denman JA, Gengenbach T, Tang P, et al. l-Leucine as an excipient against moisture on in vitro aerosolization performances of highly hygroscopic spray-dried powders. Eur J Pharm Biopharm. 2016;102:132–41.
Shekunov BY, Chattopadhyay P, Tong HHY, Chow AHL. Particle size analysis in pharmaceutics: principles, methods and applications. Pharm Res. 2007;24(2):203–27.
Holdich RG. Fundamentals of particle technology. Chapter 2 particle characterisation. Shepshed: Midland Information Technology and Publishing; 2002.
Wei G, Mangal S, Denman J, Gengenbach T, Lee Bonar K, Khan RI, et al. Effects of coating materials and processing conditions on flow enhancement of cohesive acetaminophen powders by high-shear processing with pharmaceutical lubricants. J Pharm Sci. 2017;106(10):3022–32.
Zhou Q, Denman JA, Gengenbach T, Das S, Qu L, Zhang H, et al. Characterization of the surface properties of a model pharmaceutical fine powder modified with a pharmaceutical lubricant to improve flow via a mechanical dry coating approach. J Pharm Sci. 2011;100(8):3421–30.
Nie H, Su Y, Zhang M, Song Y, Leone A, Taylor LS, et al. Solid-state spectroscopic investigation of molecular interactions between Clofazimine and Hypromellose phthalate in amorphous solid dispersions. Mol Pharm. 2016;13(11):3964–75.
Zhou QT, Loh ZH, Yu J, Sun SP, Gengenbach T, Denman JA, et al. How much surface coating of hydrophobic azithromycin is sufficient to prevent moisture-induced decrease in aerosolisation of hygroscopic amorphous colistin powder? AAPS J. 2016;18(5):1213–24.
Pilcer G, Vanderbist F, Amighi K. Spray-dried carrier-free dry powder tobramycin formulations with improved dispersion properties. J Pharm Sci. 2009;98(4):1463–75.
Wang W, Zhou QT, Sun S-P, Denman JA, Gengenbach TR, Barraud N, et al. Effects of surface composition on the aerosolisation and dissolution of inhaled antibiotic combination powders consisting of colistin and rifampicin. AAPS J. 2016;18(2):372–84.
Mangal S, Meiser F, Tan G, Gengenbach T, Denman J, Rowles MR, et al. Relationship between surface concentration of L-leucine and bulk powder properties in spray dried formulations. Eur J Pharm Biopharm. 2015;94:160–9.
Heinz A, Strachan CJ, Gordon KC, Rades T. Analysis of solid-state transformations of pharmaceutical compounds using vibrational spectroscopy. J Pharm Pharmacol. 2009;61(8):971–88.
Gandhi R, Pillai O, Thilagavathi R, Gopalakrishnan B, Kaul CL, Panchagnula R. Characterization of azithromycin hydrates. Eur J Pharm Sci. 2002;16(3):175–84.
Rajkumar BJ, Ramakrishnan V. Infrared and raman spectra of L-valine nitrate and L-leucine nitrate. J Raman Spectrosc. 2000;31(12):1107–12.
Nie H, Mo H, Zhang M, Song Y, Fang K, Taylor LS, et al. Investigating the interaction pattern and structural elements of a drug–polymer complex at the molecular level. Mol Pharm. 2015;12(7):2459–68.
Weiler C, Egen M, Trunk M, Langguth P. Force control and powder dispersibility of spray dried particles for inhalation. J Pharm Sci. 2010;99(1):303–16.
Li HY, Neill H, Innocent R, Seville P, Williamson I, Birchall JC. Enhanced dispersibility and deposition of spray-dried powders for pulmonary gene therapy. J Drug Target. 2003;11(7):425–32.
Cipolla D, Blanchard J, Gonda I. Development of liposomal ciprofloxacin to treat lung infections. Pharm. 2016;8(1):6.
Duret C, Wauthoz N, Sebti T, Vanderbist F, Amighi K. Solid dispersions of itraconazole for inhalation with enhanced dissolution, solubility and dispersion properties. Int J Pharm. 2012;428(1):103–13.
Duret C, Merlos R, Wauthoz N, Sebti T, Vanderbist F, Amighi K. Pharmacokinetic evaluation in mice of amorphous itraconazole-based dry powder formulations for inhalation with high bioavailability and extended lung retention. Eur J Pharm Biopharm. 2014;86(1):46–54.
Rennard SI, Basset G, Lecossier D, O'Donnell KM, Pinkston P, Martin PG, et al. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol. 1986;60(2):532–8.
Feng AL, Boraey MA, Gwin MA, Finlay PR, Kuehl PJ, Vehring R. Mechanistic models facilitate efficient development of leucine containing microparticles for pulmonary drug delivery. Int J Pharm. 2011;409(1–2):156–63.
Sou T, Kaminskas LM, Nguyen T-H, Carlberg R, McIntosh MP, Morton DAV. The effect of amino acid excipients on morphology and solid-state properties of multi-component spray-dried formulations for pulmonary delivery of biomacromolecules. Eur J Pharm Biopharm. 2013;83(2):234–43.
Jong T, Li J, Morton DAV, Zhou Q, Larson I. investigation of the changes in aerosolization behavior between the jet-milled and spray-dried colistin powders through surface energy characterization. J Pharm Sci. 2016;105(3):1156–63.
Vehring R. Pharmaceutical particle engineering via spray drying. Pharm Res. 2008;25(5):999–1022.
Nie H, Byrn SR, Zhou Q. Stability of pharmaceutical salts in solid oral dosage forms. Drug Dev Ind Pharm. 2017;43(8):1215–28.
Löbmann K, Grohganz H, Laitinen R, Strachan C, Rades T. Amino acids as co-amorphous stabilizers for poorly water soluble drugs–part 1: preparation, stability and dissolution enhancement. Eur J Pharm Biopharm. 2013;85(3):873–81.
Holma B. Effects of inhaled acids on airway mucus and its consequences for health. Environ Health Perspect. 1989;79:109–13.
Acknowledgments and Disclosures
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R01AI132681. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Qi (Tony) Zhou is a recipient of the Ralph W. and Grace M. Showalter Research Trust Award. The authors are grateful for the scientific and technical assistance of the Australian Microscopy & Microanalysis Research Facility at the Future Industries Institute, University of South Australia. Kind donations of RS01 DPI device from Plastiape S.p.A. and HPMC capsules from Qualicaps, Inc. are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Tony Zhou and Tonglei Li
Rights and permissions
About this article
Cite this article
Mangal, S., Nie, H., Xu, R. et al. Physico-Chemical Properties, Aerosolization and Dissolution of Co-Spray Dried Azithromycin Particles with L-Leucine for Inhalation. Pharm Res 35, 28 (2018). https://doi.org/10.1007/s11095-017-2334-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-017-2334-9