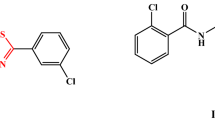
Abstract
Stemona alkaloids represent a unique class of natural products exclusively known from the three genera Stemona, Stichoneuron, and Croomia of the monocotyledonous family Stemonaceae. Structurally they are characterized by a central pyrroloazepine core usually linked with two butyrolactone rings. The great diversity, comprising 215 derivatives, is created by the formation of additional C–C linkages and oxygen bridges together with ring cleavages and eliminations of the lactone rings. Based on biosynthetic considerations they can be grouped into three structural types represented by the croomine, stichoneurine, and protostemonine skeleton. Due to the formation of characteristic terminal lactone rings and the central pyrrolidine ring pandanamine and pandamarilactonine, isolated from Stichoneuron calcicola, were suggested to represent biogenetic precursors. The taxonomically complex S. tuberosa group can be clearly segregated by the formation of stichoneurines, while the other Stemona species are characterized by protostemonine derivatives. Croomine derivatives are more widespread found in both groups and in the genus Croomia. Of chemotaxonomic significance are the different transformations of protostemonine into stemofolines with a cage-type structure or into pyridoazepines characterized by a six-membered piperidine ring. Stimulated by the great interest in the antitussive activity of Stemona alkaloids, differences in transport mechanisms and potencies via different administrative routes were investigated. Follow-up studies on the significant insecticidal activities focused on the mode of action by using biochemical and electrophysiological approaches. Screening for acetylcholinesterase inhibitory properties revealed a much higher potency for stemofoline derivatives compared to pyridoazepines, but showed also significant activity for isomers of stenine with a stichoneurine skeleton. Evaluating synergistic growth inhibitory effects with cancer chemotherapeutic agents stemofoline exhibited the most potent effect in the reversal of permeability glycoprotein-mediated multidrug resistance. The anti-inflammatory activity of some derivatives was identified as an inhibition of the expression of inflammatory mediators. Comparing the diverse bioactivities of Stemona alkaloids stemofoline-type derivatives are the most versatile compounds representing promising lead structures for further development as commercial agents in agriculture and medicine.
Similar content being viewed by others
Introduction
In the framework of UNESCO´s program “Man and the Biosphere” (MAB) broad-based phytochemical investigations within tropical plant families were carried out in our laboratory to find a replenishing source of naturally occurring insecticides or fungicides. Besides highly active sulfur-containing amides from the genus Glycosmis of the Rutaceae (Greger et al. 1996; Hofer and Greger 2000) and flavaglines from Aglaia of the Meliaceae (Brader et al. 1998; Bacher et al. 1999) we found very promising biological activities in the genus Stemona of the small monocotyledonous family Stemonaceae (Brem et al. 2002; Pacher et al. 2002). As reviewed previously the high insecticidal activity could be attributed to family-specific Stemona alkaloids (Greger 2006), whereas the antifungal property was caused by different groups of stilbenoids (Greger 2012). In addition, the accumulation of tocopherols with antioxidant activity was shown to represent another chemical character of that family (Brem et al. 2004). Apart from sporadic reports on iminosugars (Asano et al. 2005) and chlorogenic acid derivatives (Ge et al. 2007) continuative investigations mainly focused on Stemona alkaloids due to their unique molecular structures and wide range of bioactivities. Synthetic approaches to their intricate structures were summarized in a microreview by Alibés and Figueredo (2009) and more recent advances were reviewed by Liu and Wang (2015). A number of innovative strategies have been developed recently for the construction of the structurally simplest stemoamide (214) (Brito and Pirovani 2018). Moreover, extensive chromatographic comparisons of extracts from wild and cultivated Stemona species provided the basis for chemotaxonomic conclusions as well as for hypotheses regarding biosynthetic connections within this class of alkaloids (Schinnerl et al. 2007; Kongkiatpaiboon et al. 2011).
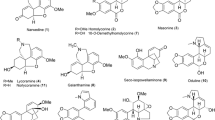
Although the basic biosynthetic steps in the formation of Stemona alkaloids are still unknown different suggestions regarding the origin of the central building blocks have been published (Seger et al. 2004; Greger et al. 2009; Wang and Chen 2014). Structural classifications were based either on pure chemical perspectives (Pilli et al. 2010) or on biosynthetic considerations (Greger 2006; Wang and Chen 2014). As a result, Stemona alkaloids were classified either into eight groups by Pilli et al. (2010), or into two classes with fourteen types by Wang and Chen (2014). By contrast, only three basic skeletons were discriminated by Greger (2006). The present review provides an updated classification of 215 Stemona alkaloids including all newly described derivatives. In spite of the many new structures the overview supports the previous classification into three basic skeletons (Tables 1, 2, 3) based on presumed biosynthetic connections and on the distribution of specific structures in related species (Fig. 1).
Three basic structures of Stemona alkaloids (Greger 2006)
In continuation of the previous review (Greger 2006) reports on bioactivties were summarized indicating remarkable progress in deciphering the mode of action. Stimulated by the great interest in the antitussive activity of Stemona alkaloids, investigations on differences in transport mechanisms and potencies via different administrative routes were carried out (Lin et al. 2008a, b; Leung et al. 2006; Zhou et al. 2009). Moreover, different ways to prepare structurally diverse analogues of active compounds were elaborated to find an efficient synthetic route (Frankowski et al. 2008, 2011). In continuation of previous investigations on insecticidal activities (Sakata et al. 1978; Jiwajinda et al. 2001; Brem et al. 2002; Kaltenegger et al. 2003) follow-up studies focused on the mode of action by using biochemical and electrophysiological approaches (Tang et al. 2008). With regard to the acetylcholinesterase inhibiting properties of Stemona alkaloids, bioassays with naturally-occurring as well as synthetically prepared derivatives informed about structure–activity relationships (Wang et al. 2007a; Baird et al. 2009; Chaiyong et al. 2010; Sastraruji et al. 2010, 2012; Ramli et al. 2013, 2014; Lai et al. 2013). Following the first report on multidrug resistance properties of an ethanolic extract of Stemona aphylla (Limtrakul et al. 2007), a series of studies evaluated the synergistic growth inhibitory effect of Stemona alkaloids with cancer chemotherapeutic agents (Chanmahasathien et al. 2011a, b; Umsumarng et al. 2013, 2015, 2017, 2018). Furthermore, their anti-inflammatory activity was investigated and regarded as an inhibition of the expression of diverse inflammatory mediators (Hosoya et al. 2011; Lim et al. 2015; Jung et al. 2016; Lee et al. 2016; Wu et al. 2018). The present review also reports on the nematicidal activity (Huang et al. 2016; Chen et al. 2018) and other miscellaneous properties as well as on the pharmacokinetics of that class of alkaloids (Dong et al. 2012; Sun et al. 2012; Han et al. 2016). Formula depictions facilitate an overview of 215 derivatives demonstrating structural relationships and biogenetic trends also serving as a guide for exploiting these compounds for further development as useful agents in agriculture and medicine.
Structural classification
Stemona alkaloids are characterized by a pyrrolo[1,2-a]azepine core (rings A, B) usually linked with two α-methyl-γ-butyrolactone rings (C, D). As shown in the structural overview (Tables 1, 2, 3) they can be derived from three basic skeletons differing in the substitution at C-9 (Fig. 1). This classification was also supported by broad-based chemotaxonomic studies within the genus Stemona indicating two clear-cut trends either towards derivatives with a stichoneurine or protostemonine skeleton (Jiang et al. 2006b; Schinnerl et al. 2007; Li et al. 2007a; Kongkiatpaiboon et al. 2011). In contrast, derivatives with a croomine skeleton were shown to be more widespread co-occurring with the other two skeletal types, and were also isolated from the genus Croomia (Noro et al. 1979; Jiang et al. 2006a; But et al. 2012).
Concerning the biosynthetic pathway the two C5-units, forming the lactone rings C and D, were previously proposed to be of terpenoid origin, and the pyrroloazepine nucleus derived from an iminium-ion-based reaction of a spermidine unit (C3NC4) (Seger et al. 2004). This hypothetical route was recently also considered by Wang and Chen (2014). However, the unexpected discovery of the secondary amine pandanamine as major constituent in the genus Stichoneuron, a sister genus of Croomia (Inthachub et al. 2009) , let expect an alternative formation of Stemona alkaloids (Greger et al. 2009). As shown in Fig. 2 the formation of the two lactone rings and the cyclization to a pyrrolidine ring in the co-occurring pandamarilactonine strongly suggests close structural relations to croomine (1). In this case the two characteristic terminal lactone rings were supposed to be derived from leucine via 4-hydroxy-4-methylglutamic acid, and the symmetrical central building block from a C4NC4 dicarboxylic acid, probably derived from two glutamic acid units (Takayama et al. 2000). Pandanamine was originally isolated from the leaves of Pandanus amaryllifolius Roxb. (Pandanaceae) (Takayama et al. 2001) and was shown to be accompanied by a series of closely related derivatives collectively called Pandanus alkaloids (Tsai et al. 2015).
Pandanamine and pandamarilactonine isolated from Stichoneuron calcicola as possible precursors of the croomine skeleton (Greger et al. 2009)
Croomines
Compounds with a croomine skeleton represent the smallest group of Stemona alkaloids comprising so far only 18 derivatives. A typical structural feature is the position of lactone ring D, which is directly attached to the azepane ring B at C-9, forming a spiro system (Table 1). Modifications are created by different oxygenations at rings B (3, 4, 13) and D (5–8, 17, 18), and the formation of an oxygen-bridge between C-6 and C-9a in the stemotinines (10–12). Tuberostemospiroline (16), tuberostemospironine (17), and stemo-lactam R (18) deviate by the loss of lactone ring C, and stemona-amine A (15) by an additional butyrolactone ring fused with the azepane ring at C-5–C-6. In the case of stemona-lactam J (13) the seven-membered azepane ring B is contracted to a six-membered piperidine-2-one ring (Hitotsuyanagi et al. 2008).
The great structural diversity of Stemona alkaloids is build up mainly by stichoneurine and protostemonine derivatives (Tables 2, 3). Important transformation steps are additional C–C linkages and oxygen-bridges either between lactone ring D and the pyrroloazepine core (Fig. 3) or within the central nucleus (Fig. 5). Further frequent modifications are created by elimination of the lactone rings, which was suggested to be the result of hydrolysis and oxidative cleavage (Fig. 4) (Lai et al. 2013), and the transformation of pyrrolidine ring A of the protostemonines into a piperidine ring (Kaltenegger et al. 2003). The latter step is accompanied by opening lactone ring C linked with chain-shortening by decarboxylation (Fig. 5).
Plausible biogenetic pathway leading to elimination of lactone ring C (redrawn from Lai et al. 2013)
Stichoneurines
Common to all stichoneurine derivatives is the ethyl group (C-16–C-17) attached at C-10. Another common trend is the frequent elimination of lactone ring C. As shown in Fig. 3 two major transformation steps (I, II) lead either to stemoninine- or to tuberostemonine-type derivatives created by an C-11–C-8 oxygen-bridge or by a C–C linkage between C-12 and C-1, respectively. In some compounds C-12 was also shown to be linked with C-9a (sessilistemonamines 119–123) or with C-8 (stemona-amines C, D (125, 126)), and C-11 linked with C-1, forming a spiro system [stemona-amine G (118), stemoxazolidinones A-C (115–117)]. A unique linkage between C-13 and C-9a was reported for sessilistemonamine D (124) (Wang et al. 2007b). Parvistemonine (37) and the related didehydroparvistemonine (38) and parvistemoline (42) deviate by an unusual oxygen-bridge between C-12 and C-16 (Lin et al. 1990, 1991a). Since these compounds are known so far only from Stemona species which are otherwise characterized by protostemonine derivatives, they were only tentatively listed in the stemoninine group (Table 2). A possible alternative formation is shown in Fig. 6.
Stemoninine-type derivatives (I)
Apart from the formation of isomers (20, 22–24, 29) structural variation of the stemoninine-type derivatives is mainly based on different oxygenations (22–24, 26, 27, 32) and dehydrogenations (26–29, 32–34, 38, 46) of the pyrrolidine ring A together with ring cleavages in 1,9a-seco-stemoenonine (35), stemona-lactam N (36), and stemona-lactam S (47). Additional modification is created by the elimination of lactone ring C (42–51) frequently leading to a 3-oxo group. An unusual linkage between C-11 and C-3 was reported for parvineostemonine (51) isolated from the stem and leaves of S. parviflora (Ke et al. 2003).
Tuberostemonine-type derivatives (II)
A higher degree of diversification is generated in derivatives of the tuberostemonine-type. Tuberostemonine (52) itself was shown to occur with many stereoisomers (53–65) and dehydro derivatives (e.g. 66–78). Additional variation is created by various oxygenations leading to hydroxy groups and oxygen-bridges, especially attached at the positions C-1, C-9, and C-9a, as well as to the formation of N-oxytuberostemonine (79). The unique structural feature of the stemoxazolidinones A-F is generated by an oxazolidin-2-one ring fused with C-1–C-9a (83, 84, 115–117) or with C-9–C-9a (85) (Hitotsuyanagi et al. 2011). A modification of the basic skeleton was reported for tuberostemoenone (87), where the six-membered ring of the tuberostemonines is contracted to a cyclopentene ring (Cui and Lin 1998). The shift of lactone ring D from C-11–C-12 to C-12–C-1, most likely produced by hydrolysis and a following relactonization of C-14 with OH-1, leads to oxotuberostemonine (88)Footnote 1 (Kondo et al. 1954; Huber et al. 1968), the related tuberostemolines A, B (101, 136) (Gao et al. 2014), and to stemona-lactam M (102) (Hitotsuyanagi et al. 2013a).
Formation of macro-rings
Oxidative ring cleavages between C-1 and C-9a or between C-9 and C-9a were considered to generate nine- (89–96) or eleven-membered macro-lactam rings (97–102, 136), respectively. Stemona-lactam G (100) is produced by a transannular hemiacetal linkage between the C-1 hydroxyl and C-9 ketone carbonyl group (Hitotsuyanagi et al. 2007a). A series of derivatives is characterized by a six membered piperidone ring A (105–113) from which tuberostemoninol (106) can be regarded as possible biogenetic precursor for derivatives with macro-rings. Tuberostemoninol (106) was also suggested to be involved in the formation of stemona-lactams K (103) and L (104), showing a rare skeleton with an unusual pyrido[1,2-a]azonine system incorporating lactone ring C (Hitotsuyanagi et al. 2010). In this case the formation of the nine-membered ring can be explained by ring cleavages between C-1 and C-9a and between C-9a and N, combined with an additional C-1–C-9 linkage. Tuberostemonone (89) was suggested to be the precursor of stemosessifoine (114) by forming a linkage between C-12 and C-9a (Guo et al. 2008). As in the stemoninine-type derivatives additional structural variation in the tuberostemonines is created by the elimination of lactone ring C (127–136).
Regarding the literature data and results from our broad-based phytochemical analyses it became apparent that actually only few derivatives listed in Table 2 were detected in chromatographic comparisons. Most likely, this discrepancy can be explained both by the formation of artifacts and by the low concentrations of some derivatives which were not detectable in chromatographic systems, especially when only small quantities of plant material were extracted. It has already been pointed out previously that especially bisdehydro-derivatives (e.g. 66–76) may be regarded as artifacts produced by extraction or fractionation (Greger 2006). This was also underlined in subsequent investigations (Jiang et al. 2006b; Schinnerl et al. 2007; Kongkiatpaiboon et al. 2011; But et al. 2012). On the other hand, regarding the relatively mild extraction conditions, isolation, and purification procedures, there was no indication for the formation of artifacts in the highly oxidized stemonatuberones A–C (91–93) and stemonatuberonol A (95) (Yue et al. 2014).
Protostemonines
The protostemonine derivatives are characterized by a methyl group (C-17) attached at C-10 and the formation of a mostly unsaturated lactone ring D linked to C-11 by a double bond. As shown in Fig. 5 two major transformations (I, II) within the pyrroloazepine nucleus lead either to stemofoline- or to oxystemokerrine-type derivatives. The complex cage-type structure of stemofoline (160) is produced by an oxygen bridge between C-8 and C-2 accompanied by an additional C–C linkage between C-7 and C-3, whereas the structure of oxystemokerrine (186) is characterized by transformation of the pyrrolidine ring A into a piperidine ring (Kaltenegger et al. 2003) containing an oxygen bridge between C-8 and C-1. Both routes show a common trend in hydrolyzing lactone ring C and splitting off C-21 by decarboxylation. As in the stichoneurine derivatives structural variation is also created by the loss of lactone ring C (194–209). However, in contrast to the stichoneurines, additional modification is generated by elimination of lactone ring D (210–215) or even of both rings in stemoamide (214) and parvistemoamide (215). Regarding the loss of the open carbon chains, derived from lactone ring C, it is interesting to note, that only the propyl rest of the pyridoazepines 191–193 was shown to be eliminated. The corresponding methyl substituent in methylstemofoline (182) appears to be produced in a different way by chain-shortening (Table 3). Similar to the stichoneurines, C-12 of lactone ring D can also be linked to C-9a or to C-8, leading either to the maistemonine- or the cochinchistemonine-type derivatives, respectively. However, the structures of both types clearly differ from the stichoneurines by the formation of a spiro system (Fig. 5). Although the structure of parvistemonine (37) shows close relations to the stichoneurine skeleton (Fig. 3), its exclusive occurrence in Stemona species, wich are otherwise characterized by protostemonine derivatives, let also expect an alternative formation (Fig. 6).
Protostemonine (137) itself was shown to occur with different oxidations (141–143, 159) and dehydro derivatives (140, 144–146, 151, 152, 159). Of special interest are the different oxygen bridges between C-8 and C-1 in oxyprotostemonine (141) and between C-6 and C-2 in neostemofoline (159), indicating possible biosynthetic connections to the major components oxystemokerrine (186) or stemofoline (160), respectively. Protostemodiol (147) was assumed to be a cleavage product formed by hydrolyzation, the same as in neostemodiol (197) (Tang et al. 2008).
Stemofoline-type derivatives (I)
Structural variation in the protostemonines is mainly created by stemofoline-type derivatives (160–182). Apart from the formation of isomers (161, 170, 171?, 174) diversification is primarily build up in the open butyl side chain attached at C-3. Hydroxylation was mainly found at C-2´ (C-19) (164, 165, 169, 180), but also at C-1′ (C-18) (163), C-3′ (C-20) (176, 177), and at the terminal C-4′ (C-22) (167, 171?). The double bonds between C-1′ and C-2′ in didehydrostemofoline (173) and in closely related derivatives (174–178) are most likely formed by dehydration. Additional oxidations lead to hydroxy groups in ring B at C-6 (6β-hydroxystemofoline (162)) and C-8 (stemoburkilline (181)), and in ring D at C-16 (16-hydroxystemofoline (172), as well as to the formation of the N-oxide 175. In stemofolinoside (178) a β-glucopyranosyl moiety is attached to C-5, representing the first glycosylated Stemona alkaloid (Sastraruji et al. 2005).
Oxystemokerrine-type derivatives (II)
Only few modifications are known from the oxystemokerrine-type derivatives characterized by a pyridoazepin basic structure. Apart from isomerism (188) and the loss of the propyl side chain (191–193) further variations are created by N-oxidation (187, 192), the cleavage of the azepan ring B (193), and the elimination of lactone ring D in oxstemokerrilactone (212) (Wang et al. 2007b). The closely related stemokerrine (183) deviates by a double bond between C-7 and C-8 and shows further modifications by N-oxidation (184, 185) and methoxylation of the propyl side chain (185). Stemokerrine (183) was proposed to be the biogenetic precursor of cochinchistemonine (189) and cochinchistemoninone (190) by opening the ether bond between C-8 and C-11 and forming a linkage between C-8 and C-12 (Lin et al. 2007a; Wang et al. 2007b).
Bioactivities
Antitussive activity
As reviewed previously, the antitussive activity of the tuberous roots of Stemona tuberosa, S. japonica, and S. sessilifolia, called Baibu in Chinese, was at all times of special importance in traditional Chinese medicine (TCM) (Greger 2006; But et al. 2012). Using the citric acid-induced guinea pig cough model Chung et al. (2003) demonstrated for the first time significant antitussive activity for the two tuberostemonine-type derivatives neotuberostemonine (59) and neostenine (129) isolated from S. tuberosa. The potency of these compounds was comparable to codeine but not involving opioid receptors in guinea pigs. Taking into account the chemical variation within the S. tuberosa group Xu et al. (2006) investigated plant samples originating from different provinces of China and found that the different alkaloid profiles could be grouped into four types represented by tuberostemonine (52), neotuberostemonine (59), stemoninine (21), or croomine (1) as major components. In spite of these chemical differences all samples demonstrated different degrees of antitussive properties, suggesting that besides tuberostemonine- (52, 59, 129) or stemoninine-type structures (21) alkaloids with a croomine skeleton (1) can also contribute to the antitussive properties. Using the same test system Lin et al. (2006) reported on significant antitussive activity of bisdehydrostemoninine (28). However, it exhibited markedly lower potency in cough suppression than neotuberostemonine (59).
After intraperitoneal and oral administration a dose-dependent inhibition was also described for the stemoninine-type derivatives stemoninine (21) and stemoninoamide (44), suggesting that both compounds have a good oral absorption (Lin et al. 2008a). Similarly, no significant difference in activity between the two routes of application was determined for croomine (1) (Lin et al. 2008b). In a more detailed study the intestinal absorption of neotuberostemonine (59) and neostenine (129) was investigated using a Caco-2 monolayer model. Both compounds exhibited good absorptive permeability and thus are likely to be orally active components. Furthermore, both alkaloids were identified to be the substrates of P-glycoprotein (Leung et al. 2006). Oral absorption and antitussive activities were compared between the stereoisomers tuberostemonine (52), neotuberostemonine (59), and tuberostemonine H (60). All three isomers exhibited dose-dependent inhibitory effects after intraperitoneal administration. Tuberostemonine (52) itself was shown to have the same potency via both oral and intraperitoneal dosing, whereas neotuberostemonine (59) exhibited significantly lower, and tuberostemonine H (60) no oral activity. The activity of a further isomer, tuberostemonine J (61), could not be tested due to scarce material, but it demonstrated a moderate permeability in Caco-2 monolayer cells. The differences in transport mechanisms and potencies of the antitussive activity via different administrative routes were discussed as a result of various stereo configurations (Zhou et al. 2009).
A follow-up study confirmed the antitussive effects of the four major alkaloids croomine (1), stemoninine (21), tuberostemonine (52), and neotuberostemonine (59) of S. tuberosa when their potency was compared at a fixed dosage. Moreover, it was shown that they actually do not express their effects through the same mechanisms. Whereas the stichoneurine derivatives 21, 52, and 59 acted on the peripheral cough reflex pathway, croomine (1) was shown to have central depressant effects. This may partly account for the adverse side effects previously reported for using corresponding plant samples (Xu et al. 2010). However, all results mentioned above disclosed only chemicals responsible for the antitussive activity of S. tuberosa but not for that of S. japonica and S. sessilifolia, the other two representatives of the Chinese drug Baibu, whose chemical constituents differ greatly by the accumulation of protostemonine derivatives. Yang et al. (2009) closely analyzed the alkaloid composition of S. sessilifolia and investigated the antitussive activity of the main constituents protostemonine (137), maistemonine (153), and the croomine-type derivative stemospironine (4) also using the citric acid-induced cough model. All three alkaloids showed significant antitussive activity following peripheral administration. Stemonamine (206), which is structurally closely related to 153, also showed activity after administration through a single intracerebroventricular injection. Due to the considerable interest in the synthesis of structurally diverse analogues of biologically active compounds Frankowski et al. (2008) elaborated an efficient synthetic route for the exploration of tuberostemonine-type analogues with a tricyclic core. In a following study they reported on the synthesis and receptor profiling of these analogues which reveal a potent class of sigma ligands (Frankowski et al. 2011).
Insecticidal activity
On the basis of chronic feeding bioassays with neonate larvae of the polyphagous pest insect Spodoptera littoralis (Lepidoptera, Noctuidae) reared on artificial diet the leaf and root extract of Stemona collinsiae displayed very high insect toxicity. By contrast, the extracts from S. tuberosa demonstrated low activity in roots and no activity in leaves (Brem et al. 2002). In accordance with previous findings with the diamondback moth Plutella xylostella (Jiwajinda et al. 2001) this strong activity of S. collinsiae could be attributed to didehydrostemofoline (173), whose LC50 value at 0.84 ppm was higher than that of the co-occurring stemofoline (160) with 2.04 ppm and the closely related 2′-hydroxystemofoline (164) with 30.33 ppm. Clearly different was the activity of tuberostemonine (52) from the roots of S. tuberosa, exhibiting a LC50 value at > 500 ppm. In addition to the activities after oral administration the crude extracts of S. tuberosa and S. collinsiae also showed strong feeding inhibitory properties against fifth instar larvae in a leaf disk choice test. In view of the low toxicity of S. tuberosa, the high repellent effect of the root extract was surprising. Further experiments led to the conclusion that tuberostemonine (52) acts as a potent repellent without toxic effects (Brem et al. 2002). In view of these results it can be assumed that the recently published significant insecticidal activity of the root extract of S. collinsiae against the fly Parasacrophaga ruficornis (Sakulpanich et al. 2017) can also be attributed to didehydrostemofoline (173), which was shown to be the main component of all samples of S. collinsiae collected in Thailand (Kongkiatpaiboon et al. 2011).
Comparing the insecticidal properties of the crude extracts of four other Stemona species, S. curtisii and S. cochinchinensis proved to be most active, whereas S. kerrii and S. aphylla (published as unknown species HG 915) clearly showed less activity. The high activity of the first two species was attributed to stemofoline (160), and was assumed to be caused by neurotoxic interactions resulting in uncontrolled hyperactivity of larvae. In contrast to this excitatory effect causing immediate death, the extracts of the two latter species displayed a different mode of action characterized by a delayed entrance of death accompanied by softening of the larval bodies. The latter phenomenon has already been described for stemospironine (4) (Sakata et al. 1978). The main causative agents for S. kerrii were identified as the pyridoazepine oxystemokerrine (186) with a LC50 value at 5.9 ppm and the pyrroloazepine dehydroprotostemonine (140) at 6.1 ppm (Kaltenegger et al. 2003). In view of the observed infraspecific chemical variation in S. curtisii (Schinnerl et al. 2007; Kongkiatpaiboon et al. 2011), ten root samples collected from various localities in Thailand were analyzed by HPLC and the four major components stemofoline (160), stemocurtisine (191), oxystemokerrine (186), and stemocurtisinol (188) were investigated for their insecticidal activities. In accordance with previous results the highest activity was determined for 160 (Kongkiatpaiboon et al. 2013).
The stemofolines 160 and 173 were shown to display rapid reactions leading to complete cessation of food intake. Since this reaction was accompanied by vomiting and trembling, it was interpreted as an expression of toxicity and not simply as a feeding inhibition (Brem et al. 2002). The strong activity of stemofoline (160) was first detected in the leaf extract of S. japonica, where its toxicity was shown to be much higher than the co-occurring croomine-type derivative stemospironine (4), showing a different mode of action (Sakata et al. 1978). Similar to commercialized neonicotinoids the mode of action of 160 was determined to be agonist at insect nicotinic acetylcholine receptors by using electrophysiological in vitro tests and by in vivo screenings against relevant agricultural insect pests (Tang et al. 2008). It was found that 160 and the structurally related derivatives 6β-hydroxystemofoline (162), 16-hydroxystemofoline (172), and neostemofoline (159) tested in vitro are active on isolated neurons from the pest insect Heliothis virescens. For the 6-hydroxy derivative 162 an EC50 value of 157 nmol indicated lower potency than 160 with 50 nmol, whereas hydroxylation at the lactone ring D in 172 showed an even stronger effect than 160 with an EC50 value at 12 nmol. By contrast, 13-demethoxy-11S,12R-dihydroprotostemonine (149) (later also named isostemocochinin (Yi et al. 2015)) and protostemodiol (147) were shown to act as antagonists. This study has shown that the cage-type moiety of stemofolines is pivotal to their insecticidal activity (Fig. 7). The half-cage derivative 159 is a partial agonist due to the significant changes in the cage moiety (Tang et al. 2008). Stemofoline derivatives with their high insecticidal activity already inspired as lead structures the development of synthetic cyanotropanes, a novel class of commercial insecticides interacting at the nicotinic acetylcholine receptor (Lind et al. 2002).
Structure–activity relationships also became apparent within the pyridoazepine derivatives displaying the highest activity for oxystemokerrine (186), containing an oxygen bridge between C-1 and C-8, with a LC50 value at 5.9 ppm. N-oxidation in oxystemokerrine-N-oxide (187) or replacement of the oxygen bridge by a double bond in stemokerrine (183) diminished activities to LC50 values at 12.5 ppm for 187 and 58 ppm for 183. A significant decrease of toxicity, however, was caused by the loss of the open butyl side chain in stemocurtisine (= pyridostemin) (191) (LC50 = 149 ppm) or its O-methylation in methoxystemokerrine-N-oxide (185) (LC50 = > 100 ppm). Somewhat different activities against mosquito larvae (Anopheles minimus) were reported for stemocurtisinol (188), a stereoisomer of 186, with LC50-values at 39 ppm and for 191 at 18 ppm (Mungkornasawakul et al. 2004a). Furthermore, comparing all results mentioned above, it became apparent that the double bond between C-11 and C-12 of lactone ring D, characteristic for many protostemonine derivatives (Table 3), also plays a crucial role. It is missing in stemocochinine (148) and parvistemonine (37), where it led to a strong decrease of activity with LC50 values at 170 ppm for 148 or even > 200 ppm for 37 (Kaltenegger et al. 2003).
Nematicidal activity
In a more recent investigation twenty-five Stemona alkaloids were isolated from the roots of S. parviflora, collected in Hainan island in China, and tested for their activity against the nematode Panagrellus redivivus. The activity of all isolated compounds was evaluated in a lethality assay showing IC50 values at 42.5, 1.95, and 76.4 µmol for 3β-n-butylstemonamine (157), protostemonamide (194), and (+) oxystemofoline (167), respectively, comparable to the positive control albendazole with 67.2 µmol. However, protostemonine (137) and stemofoline (160) showed nematicidal activity superior to that of the control with IC50 values at 0.10 and 0.45 µmol, respectively (Huang et al. 2016). Nematicidal activity was recently also reported for the crude extract of S. mairei, which is consistent with the presence of protostemonine (137) as major constituent (Chen et al. 2018).
Acetylcholinesterase inhibition
Since the insecticidal properties of Stemona alkaloids were found to be associated with acetylcholinesterase (AChE) inhibition various derivatives were screened for their activities in bioassays using huperzine A, physostigmine or galanthamine as positive controls. Wang et al. (2007a) reported for the first time on AChE-inhibiting activity of Stemona alkaloids. From the diastereoisomeric tuberostemonine-type alkaloids sessilistemonamines A–C (119, 121, 120) only A (119) and B (121) were found to be moderately active with IC50 values at 68.8 and 17.1 µmol, respectively, whereas C (120) did not show any activity.
Five naturally occurring stemofoline-type alkaloids (166, 173, 176, 177, 182) together with the two synthesised stereoisomeric derivatives (3′R)-hydroxystemofoline and (11E)-methylstemofoline were screened for their AChE-inhibiting properties by TLC bioautography. It was found that didehydrostemofoline (173) and (3′S)-hydroxystemofoline (166) were clearly the most active (Baird et al. 2009). By contrast, the pyridoazepine alkaloids stemocurtisinol (188), stemocurtisine (191) and four synthetic analogues as well as the pyrroloazepine oxyprotostemonine (141) where shown to be 10–20 times less active (Chaiyong et al. 2010). Starting with the highly active 173 the same working group prepared a number of related analogues with modifications in the C-3 butyl side chain and screened their AChE inhibitory activities. They found that (1′R) hydroxystemofoline (163) was also very active, even slightly more than 173 (Fig. 7) (Sastraruji et al. 2010). In a follow-up study thirty-two stemofoline analogues were prepared from 173, comprising several novel C-3 side-chain amino, carbamate, triazole and oxazole derivatives. Compared to earlier results, this study showed that in general the amine derivatives were more active than the previously reported alcohol derivatives. Preliminary molecular docking studies indicated that derivatives with small side chains bind in a differnt way to the binding sites of AChE than those with longer side chains. Using the TLC bioautographic method, didehydrostemofoline (173) was shown to be the most active of the tested compounds against human (h) and electric eel (ee) AChE. However, the activity was significantly less than that of the posititive control galanthamine (Sastraruji et al. 2012).
Comparing the AChE-inhibiting properties of seven stichoneurine derivatives, isolated from the roots of Stichoneuron halabalensis, significant activity against hAChE was reported for stemoninine (21) and bisdehydrostemoninine A (34) with IC50 values at 3.74 and 5.52 µmol, respectively. Stichoneurine E (24) was shown to be the only derivative with high activity against eeAChE with 5.90 µmol. All other compounds were less active than the positive control galanthamine with 0.54 µmol against hAChE and 0.32 µmol against eeAChE (Ramli et al. 2013). Structure–activity relationship became apparent for four further stichoneurine derivatives, isolated from the roots of Stichoneuron caudatum, when tested against hAChE. The isomeric sessilistemonamines E (123) and F (122) exhibited significant activity for 123 with an IC50 value at 9.1 µmol, but no activity (> 100 µmol) for 122. Moreover, a modest activity at 71.5 µmol was determined for stichoneurine F (40), while the closely related stichoneurine G (41) was not active at 100 µmol, indicating an adverse effect by the additional C-9 hydroxy group (Ramli et al. 2014).
A bioactivity-guided chromatographic fractionation of the root extract of S. tuberosa (erroneously published as S. sessilifolia) led to the isolation of five stichoneurine derivatives, from which stenine (127) and especially stenine BFootnote 2 (128) exhibited strong activity with IC50 values at 19.8 and 2.1 µmol, respectively, while neotuberostemonine (59), neostenine (129), and stenine A (130) showed only very weak activity. Since the compounds 127, 128, and 129 are stereoisomers, the observed differences in the inhibition of AChE could only be explained by different spatial configurations. Moreover, N-oxidation in 130 appeared to be very disadvantaged for activity. A kinetic analysis of 128 indicated that its AChE inhibition was reversible and competitive. In addition, a docking program was applied to predict the binding modes of 128 into the huperzine A binding site of AChE. It was shown that 128 acts internally around the tryptamine 84 and has the same hydrogen bonding interactions with tyrosine 130 as huperzine A. Regarding the suggested role of AChE in the pathogenesis of Alzheimer´s disease, stenine B (128) was considered as a new interesting drug candidate (Lai et al. 2013).
Multidrug resistance reversing properties
The development of multidrug resistance (MDR) is a major impediment to the chemotherapeutic treatment of many forms of human cancer. A major factor in this process is the overexpression of the “permeability glycoprotein“(P-gp) in tumor cells preventing sufficient accumulation of anticancer dugs thereby avoiding their cytotoxic or apoptotic effects. The ethanolic crude extract of Stemona aphylla (published as S. curtisii) was shown to modulate P-gp activity and was expected to be effective in the treatment of multidrug-resistant cancers (Limtrakul et al. 2007). Stimulated by these findings a more detailed study aimed to obtain the MDR-reversing components of Stemona species. Bioassay-guided fractionation led to the isolation of oxystemokerrine (186) and stemocurtisine (191) from S. aphylla, and stemofoline (160) from S. burkillii. They were evaluated for synergistic growth inhibitory effect with cancer chemotherapeutic agents including vinblastine, paclitaxel, and doxorubicin of KB-V1 (multidrug resistance cervical carcinoma cell line) cells using verapamil as a comparative agent. Among the three alkaloids stemofoline (160) exhibited the most potent effect in vitro in the reversal of P-gp-mediated MDR (Chanmahasathien et al. 2011a). In a following investigation it was shown that 160 could increase the accumulation and retention of radiolabeled drugs or fluorescent P-gp substrates in a dose-dependent manner and that it may interact directly with P-gp and inhibit P-gp activity, whereas it has no effect on P-gp expression (Chanmahasathien et al. 2011b). The inhibition of P-gp mediated MDR was later also examined in KB-V1 and K562/Adr (human leukemic) cell lines with twelve other stemofoline derivatives, including didehydrostemofoline (173) and eleven synthesised derivatives with different OH- and N-substitutions in the C-3 side-chain. The results demonstrated structure–activity relationships and revealed that 173 and two N-substituted derivatives dramatically increased the intracellular accumulation and cytotoxicity of the antineoplastic drugs in the P-gp overexpressing KB-V1 and K562/Adr cell lines in vitro (Umsumarng et al. 2013). Since the mode of action of the stemofolines on P-gp inhibition was unclear a continuing study aimed to investigate whether the stemofolines can reverse MDR through the inhibition of P-gp function or expression in human MDR leucemic cells (K562/Adr), or both. Since the stemofolines did not alter the P-gp expression, as determined by Western blotting, it was concluded that they reverse MDR via the inhibition of P-gp function (Fig. 7) (Umsumarng et al. 2015). The same authors tested eight naturally occurring protostemonine derivatives for their MDR modulation activity. They found that the three stemofoline-type derivatives isostemofoline (161), didehydrostemofoline (173), and isodidehydrostemofoline (174), having a core caged ring structure with non-polar side chains, enhanced the chemotherapeutic sensitivity of MDR leukemic K562/Adr cells, which overexpressed P-gp. For pharmaceutical safety testing they were shown to be not toxic to normal human fibroblasts and peripheral blood mononuclear cells and did not induce hemolysis in either human or rat erythrocytes (Umsumarng et al. 2017). More recent results indicated that stemofolin (160) itself did not affect tumor growth, but was able to enhance the efficacy of doxorubicin in scid-beige mice bearing K562/Adr xenografts. Additionally, the combination therapy significantly reduced the Ki67proliferation marker and induced apoptosis (TUNEL) (Umsumarng et al. 2018).
Anti-inflammatory activities
The methanolic root extract of Stemona javanica showed anti-inflammatory activity as measured by inhibition of nitric oxide (NO) production in lipopolysaccharide (LPS)-stimulated J774.1 mouse macrophage-like cells. Bioassay-guided fractionation led to the isolation of two active compounds, the alkaloid stemofoline (160) and the stilbenoid stemanthrene C. The inhibition mechanism of 160 was proposed to suppress inducible NO synthase in the macrophage cells stimulated by LPS, whereas that of stemanthrene C was due to radical scavenging (Hosoya et al. 2011). The anti-inflammatory effect of the water extract of S. tuberosa was investigated in LPS-stimulated RAW 264.7 macrophages and in cigarette smoke-induced lung inflammation mouse models. The results suggested that the extract attenuated pulmonary inflammatory responses by inhibiting the expression of diverse inflammatory mediators. Preliminary HPLC–ESI–MS analysis let expect that stichoneurine derivatives with stemoninine (21) as major component together with croomine (1) were responsible for the anti-inflammatory effect (Lim et al. 2015). In a following study it was shown that tuberostemonine N (56) significantly inhibited the secretion of proinflammatory cytokines and chemokines in bronchoalveolar lavage fluid and decreased alveoli size in lung tissue (Jung et al. 2016). A reduction of LPS-induced NO was also observed with a methanolic root extract of S. tuberosa in murine BV2 microglia cells. Bioactivity-guided fractionation led to the isolation of eight alkaloids from which bisdehydroneotuberostemonine (67), epi-bisdehydroneotuberostemonine J (72), and bisdehydrotuberostemonine (66) showed significant inhibitory effects (Lee et al. 2016).
A more recent study reported on protostemonine (137) as the main anti-inflammatory alkaloid from the roots of S. sessilifolia. The results demonstrated that 137 effectively attenuated LPS-induced inflammatory responses in vitro and in vivo. The effects were shown to be associated with the decreased phosphorylation of MAP (mitogen-activated protein) kinase and proteinkinase B and the reduced expression of pro-inflammatory mediators. The results suggested that 137 may be a potential therapeutic agent in the treatment of acute lung injury (Wu et al. 2018).
Pharmacokinetics
A rapid and selective UPLC-HDMS method (ultra-performance liquid chromatography coupled with high-resolution mass spectrometry) was developed to analyse the active alkaloids of Stemona extracts and their metabolites in rat plasma following oral administration. Twenty-nine compounds in “Radix Stemonae” extract and fourty-seven compounds in rat plasma samples were detected in a single injection within 21 min, comprising croomine and stichoneurine derivatives. The study showed that hydroxylation, hydration, reduction, and change of alkenes to dihydrodiol were the crucial metabolic transformation pathways (Dong et al. 2012). An UPLC-QTOF (quadrupole time-of-flight)-HDMS method was successfully applied to investigate the pharmacokinetics of croomine (1), neotuberostemonine (59), and tuberostemonine (52) after oral administration of “Radix Stemonae” extract. The extract was rapidly distributed and then eliminated from rat plasma. The biodistribution of 59 and 52 showed higher levels in liver and lung. Croomine (1) was detected in brain and showed that it could cross the blood–brain barrier, indicating that it has an antitussive effect by acting on the central nervous system. By contrast, neotuberostemonine (59) and tuberostemonine (52) were not detected in brain, demonstrating that they act at the peripheral cough reflex pathway (Sun et al. 2012). A rapid and sensitive LC–ESI–MS/MS (liquid chromatography/electrospray ionization tandem mass spectrometry) method was developed and validated for determining protostemonine (137) in rat plasma. After oral administration 137 was rapidly absorbed from the gastrointestinal tract and has shown dose-independent pharmacokinetic profiles. Moreover, a total of 10 metabolites were identified in rat urine. Hydrolysis and oxygenation were proposed to be the major metabolic pathway (Han et al. 2016).
Miscellaneous properties
The crude extract of S. tuberosa enhanced apoptosis of medullary thyroid cancer cells (MTC), whereas that of S. collinsiae only moderately increased the apoptotic effect (Rinner et al. 2004). In a following study four different extracts from S. tuberosa with n-hexane, dichloromethane, ethyl acetate, and methanol were examined for their antiproliferative effects in two MTC cell lines. Only the dichloromethane extract was shown to inhibit cell growth and viability in a dose dependent manner. Moreover, it also exhibited strong apoptotic effects. Chromatographic analyses let expect that croomine (1) and/or tuberostemonine-type derivatives are responsible for these activities (Li et al. 2007b).
Phuwapraisirisan et al. (2006) investigated the effect of stemofoline-type alkaloids on the delay of parturation of Wistar rats. Since preliminary results suggested that they may block uterine contraction regulated by oxytocin released during last pregnancy, the oxytocin antagonistic effects of didehydrostemofoline (173) (published as asparagamine A), stemofoline (160) and 2′-hydroxystemofoline (164) were examined on delayed parturation in pregnant rats. The results showed that 173 significantly delayed parturation, while no delay was observed for 160 and 164. However, it could not be confirmed that parturation delayed by 173 is due to its competitive binding to oxytocin receptor.
Synthetic intermediates of stemonamide (207) were reported to inhibit in vitro activities of Pim-3 kinase, a protooncogene with serine/threonine kinase activity, and its related kinases. The alkaloids were also shown to inhibit cell proliferation of various human pancreatic, hepatocellular and colon cancer cell lines, and to induce apoptosis of human pancreatic cells in vitro. The results indicated that these compounds and related derivatives may be effective for the treatment of tumors of endoderm-derived organs, particularly the pancreas (Li et al. 2010).
The extract of S. tuberosa was tested for activity against neuroaminidase of H5N1 avian virus in silico. From eighty-one compounds, including Stemona alkaloids and stilbenoids, thirty-three were selected as possible lead structures according to toxicity tests and Lipinski´s rule of five. In docking analysis croomine (1) exhibited a promising activity by interacting to the active site of H5N1 neuroaminidase (Manohar 2013).
Protostemonine (137) was shown to exhibit protective effects on LPS/D-galactosamine (GalN)-induced acute liver failure, a clinical syndrom with a very high mortality rate. It was also found that induction of the hepatic antioxidant enzyme HO-1 plays an important role in the hepatoprotective activity of 137. Moreover, the study has shown, that even at the highest utilized dosages 137 exhibited undetectible toxic effects on vital organs of mice (Cheng et al. 2015).
Idiopathic pulmonary fibrosis (IPF) is the most common and lethal lung disease characterized by a progressive and irreversible decline in lung function. Xiang et al. (2016) investigated the effects of neotoberostemonine (59) on bleomycin (BLM)-induced lung fibrosis in mice and the underlying mechanism. The study revealed that 59 significantly ameliorated lung histopathological changes and decreased inflammatory cell counts in the bronchoalveolar lavage fluid. The results suggested that the protective effects of 59 for BLM-induced mice was equivalent to that of nintedanib, a new drug for IPF.
Chemotaxonomy
Stemona alkaloids represent a typical chemical character of the family Stemonaceae so far not detected in any other plant family. They are mainly accumulated in the tuberous roots of Stemona species, but were also isolated from leaves and stems and the non-tuberous roots and rhizomes of the two other genera of the family Stichoneuron and Croomia. With regard to the many new data it became obvious that in accordance with previous considerations the taxonomically complex S. tuberosa group can be clearly distinguished from the other Stemona species by the formation of stichoneurine alkaloids (Greger 2006; Zhou et al. 2006; Jiang et al. 2006a, b; Schinnerl et al. 2007; Kongkiatpaiboon et al. 2011; But et al. 2012) (Fig. 3). With the formation of stemoninine-type derivatives this biogenetic trend was also detected in the two closely interrelated Stichoneuron species S. caudatum (Schinnerl et al. 2005; Ramli et al. 2014) and S. halabalensis (Ramli et al. 2013). However, in contrast to Stemona species no tuberostemonine-type alkaloids were found, characterized by the C-12–C-1 cyclization. Instead, both species deviate by the formation of the rare C-12–C-9a cyclization in the sessilistemonamines F and E (122, 123). It is noteworthy, that here no croomine derivatives (Table 1) were detected, which are otherwise widely distributed in the genera Stemona and Croomia. Hence, it is tempting to assume that pandanamine and pandamarilactonine, isolated from the rare Stichoneuron calcicola (Greger et al. 2009), represent possible precursors for the formation of the croomine skeleton (Fig. 2).
Within the S. tuberosa group distinct trends towards different isomers of tuberostemonine (52) together with floral characters contribute to a more natural species delimitation. In Thailand the accumulation of tuberostemonine A (53) distinguishes S. phyllantha from S. tuberosa, which is characterized by neotuberostemonine (59) and tuberostemonine N (56) (Schinnerl et al. 2007). This segregation was partly also underlined in 15 accessions recently investigated in southern China. The overview showed samples with distinct accumulation trends towards the abovementioned isomers as well as to a few intermediates. In addition, some accessions deviated by a predominance of stemoninine (21). Floral characters suggested here a closer relatioship to S. tuberosa (Chen et al. 2017).
The other Stemona species are characterized by protostemonine (137) derivatives (Fig. 5). Broad-based chromatographic comparisons of root extracts from 13 different species revealed characteristic accumulation trends either towards pyridoazepine- or stemofoline-type derivatives, which appeared to be of chemosystematic interest (Schinnerl et al. 2007; Kongkiatpaiboon et al. 2011) (Fig. 8). All samples of S. collinsiae collected in different localities of Thailand uniformly showed a predominance of didehydrostemofoline (173), indicating a species-specific chemical character. The closely related S. burkillii can be distinguished by an accumulation of stemofoline (160) (Mungkornasawakul et al. 2004b; Schinnerl et al. 2007). By contrast, the alkaloid profiles of S. kerrii were characterized by pyridoazepine derivatives, especially by a predominance of stemokerrine (183). This rare trend was also detected in S. saxorum (Wang et al. 2007c), which was regarded as a synonym of S. kerrii (Ji and Duyfjes 2000). Whereas these species were shown to be characterized by distinct accumulation trends, S. curtisii exhibited infraspecific chemical variability comprising samples accumulating either stemofoline- or pyridoazepine-type derivatives, as well as those with intermediate profiles (Schinnerl et al. 2007; Kongkiatpaiboon et al. 2011). In this connection it should be pointed out that S. curtisii, a species widely distributed in southern Thailand, also deviated by its chromosome number 2n = 13–16, whereas the other Stemona species were shown to have the same chromosome number 2n = 14 (Oginuma et al. 2001; Hartl and Kiehn 2004).
Pronounced chemical differences were also observed in S. aphylla, demonstrating infraspecific variability between different localities as well as different individuals within the same population. Generally, the results indicated a dominating trend towards accumulation of the pyridoazepine derivative oxystemokerrine (186), frequently accompanied by the structurally closely related stemocurtisine (191). This trend was also found in S. involuta supporting the suggested relationship with S. aphylla (Inthachub et al. 2010). Of some interest for chemotaxonomic conclusions was the variable formation of oxystemokerrine N-oxide (187), which might be explained by different ways of sample preparation, as well as a general reduction of alkaloid formation in some accessions, showing only small amounts of protostemonine (137) accompanied by an accumulation of stilbenoids. A mutual accumulation of alkaloids or stilbenoids was also reported for different plant parts in one sample of S. curtisii (Schinnerl et al. 2007).
On the basis of the available results it became apparent that the alkaloid profiles of the Chinese species S. sessilifolia (Yang et al. 2009), S. japonica (Tang et al. 2008; Yi et al. 2015), S. parviflora (Huang et al. 2016), and S. mairei (Chen et al. 2018) deviate from the abovementioned species by a predominance of protostemonine (137) itself, accompanied by the structurally similar maistemonine (153), protostemonamide (194), stemonamide (207), and stemonine (211) together with corresponding isomers (Fig. 8). In this group of species stemofoline-type derivatives were shown to be accumulated only in the aerial parts of S. japonica (Sakata et al. 1978; Schinnerl et al. 2007; Tang et al. 2008), and were only found as minor components in the roots of S. parviflora (Huang et al. 2016) and S. mairei (Chen et al. 2018). However, no pyridoazepine alkaloids have been detected so far in the four species. Only small amounts of protostemonine were detected in S. pierrei and S. rupestris, two closely related endemic species of Thailand. With an additional accumulation of stilbenoids they formed a chemical trend similar to that found in two samples of S. aphylla, probably indicating a reduced enzymatic activity of alkaloid formation (Kostecki et al. 2004; Kongkiatpaiboon et al. 2011).
Considering the clear-cut chemical segregation of the S. tuberosa group from the other Stemona species by the formation of stichoneurine derivatives, the many reports on that type of alkaloids in S. sessilifolia appear to be the result of not properly identified plant material. In fact, Hitotsuyanagi et al. (2013a) later corrected the name S. sessilifolia to S. tuberosa in their papers based on subsequent DNA sequence analyses. In order to avoid confusion they renamed the originally published trivial names sessilifoliamides A-L to stemona-lactams A-L, and sessilifoliamine A to stemona-amine A (Table 2). With the shrubby habit and erect stem together with the whorled shortly petiolate or sessile leaves S. sessilifolia and the assumedly conspecific S. shandongensis (Li and Fu 2007) can be distinguished from the other species which are characterized by a climbing habit and petiolate leaves. It is tempting to assume that the predominance of protostemonine (137) itself, accompanied only by structurally closely related derivatives, represents a more primitive biogenetic trend. Data from various DNA regions of different Stemona species, summarized and interpreted by But et al. (2012), largely supported the grouping outlined above (Fig. 8).
Notes
References
Alibés R, Figueredo M (2009) Strategies for the synthesis of Stemona alkaloids. Eur J Org Chem 15:2421–2435
Asano N, Yamauchi T, Kagamifuchi K, Shimizu N, Takahashi S, Takatsuka H, Ikeda K, Kizu H, Chuakul W, Kettawan A, Okamoto T (2005) Iminosugar-producing Thai medicinal plants. J Nat Prod 68:1238–1242
Bacher M, Hofer O, Brader G, Vajrodaya S, Greger H (1999) Thapsakins: possible biogenetic intermediates towards insecticidal cyclopenta[b]benzofurans from Aglaia edulis. Phytochemistry 52:253–263
Baird MC, Pyne SG, Ung AT, Lie W, Sastraruji T, Jatisatienr A, Jatisatienr C, Dheeranupattana S, Lowlam J, Boonchalermkit S (2009) Semisynthesis and biological activity of stemofoline alkaloids. J Nat Prod 72:679–684
Brader G, Vajrodaya S, Greger H, Bacher M, Kalchhauser H, Hofer O (1998) Bisamides, lignans, triterpenes, and insecticidal cyclopenta[b]benzofurans from Aglaia species. J Nat Prod 61:1482–1490
Brem B, Seger C, Pacher T, Hofer O, Vajrodaya S, Greger H (2002) Feeding deterrence and contact toxicity of Stemona alkaloids—a source of potent natural insecticides. J Agric Food Chem 50:6383–6388
Brem B, Seger C, Pacher T, Hartl M, Hadacek F, Hofer O, Vajrodaya S, Greger H (2004) Antioxidant dehydrotocopherols as a new chemical character of Stemona species. Phytochemistry 65:2719–2729
Brito GA, Pirovani RV (2018) Stemoamide: total and formal synthesis. A review. Org Prep Proc Int 50:245–259
But PPH, Shaw PC, Lin G, Jiang RW, Xu YT (2012) Authentication and quality assesssment of the antitussive herb Baibu (Radix Stemonae). In: Shyur LF, Lau ASY (eds) Advances in Botanical Research, vol 62. Elsevier, Amsterdam
Cai XH, Luo XD (2007) Alkaloids from Stemona mairei. Planta Med 73:170–173
Chaiyong S, Jatisatienr A, Mungkornasawakul P, Sastraruji T, Pyne SG, Ung AT, Urathamakul T, Lie W (2010) Phytochemical investigations of Stemona curtisii and synthetic studies on stemocurtisine alkaloids. J Nat Prod 73:1833–1838
Chanmahasathien W, Ampasavate C, Greger H, Limtrakul P (2011a) Stemona alkaloids, from traditional Thai medicine, increase chemosensitivity via P-glycoprotein-mediated multidrug resistance. Phytomedicine 18:199–204
Chanmahasathien W, Ohnuma S, Ambudkar SV, Limtrakul P (2011b) Biochemical mechanism of modulation of human P-glycoprotein by stemofoline. Planta Med 77:1990–1995
Chen G, Brecker L, Felsinger S, Cai XH, Kongkiatpaiboon S, Schinnerl J (2017) Morphological and chemical variation of Stemona tuberosa from southern China—evidence for heterogeneity of this medicinal plant species. Plant Biol 19:835–842
Chen G, Brecker L, Sandler KM, Cai XH, Kongkiatpaiboon S, Valant-Vetschera K, Schinnerl J (2018) Phytochemical characterization of the Chinese endemic species Stemona mairei and five other Stemona species. Phytochem Lett 28:168–173
Cheng DL, Guo J, Chu TT, Röder E (1988) A study of Stemona alkaloids, III. Application of 2D-NMR spectroscopy in the structure determination of stemoninine. J Nat Prod 51:202–211
Cheng Z, Yue L, Zhao WH, Yang XH, Shu GW (2015) Protective effects of protostemonine on LPS/GalN-induced acute liver failure: roles of increased hepatic expression of heme oxygenase 1. Int Immunopharmacol 29:798–807
Chung HS, Hon PM, Lin G, But PPH, Dong H (2003) Antitussive activity of Stemona alkaloids from Stemona tuberosa. Planta Med 69:914–920
Cui YX, Lin WH (1998) 2D NMR studies on tuberostemoenone and tuberostemonone. Chin J Magn Res 15:515–520
Dong W, Wang P, Meng XC, Sun H, Zhang A, Wang WM, Dong H, Wang XJ (2012) Ultra-performance liquid chromatography-high-definition mass spectrometry analysis of constituents in the root of Radix Stemonae and those absorbed in blood after oral administration of the extract of the crude drug. Phytochem Anal 23:657–667
Dong JL, Yang ZD, Zhou SY, Yu HT, Yu HT, Yao XJ, Xue HY, Shu ZM (2017) Two Stemona alkaloids from Stemona sessilifolia (Miq.) Miq. Phytochem Lett 19:259–262
Edwards OE, Feniak G, Handa KL (1962) The alkaloids of Stemona sessilifolia. Can J Chem 40:455–462
Frankowski KJ, Neuenswander B, Aubé J (2008) Explorations of Stemona alkaloid-inspired analogues: skeletal modification and functional group diversification. J Comb Chem 10:721–725
Frankowski KJ, Setola V, Evans JM, Neuenswander B, Roth BL, Aubé J (2011) Synthesis and receptor profiling of Stemona alkaloid analogues reveal a potent class of sigma ligands. Proc Natl Acad Sci USA 108:6727–6732
Fukaya H, Hitotsuyanagi Y, Aoyagi Y, Shu Z, Komatsu K, Takeya K (2013) Absolute structures of stemona-lactam S and tuberostemospiroline, alkaloids from Stemona tuberosa. Chem Pharm Bull 61:1085–1089
Gao Y, Wang J, Zhang CF, Xu XH, Zhang M, Kong LY (2014) Seven new alkaloids from the roots of Stemona tuberosa. Tetrahedron 70:967–974
Ge F, Ke CQ, Tang W, Yang XZ, Tang CP, Qin GW, Xu RS, Li TX, Chen XW, Zuo JP, Ye Y (2007) Isolation of chlorogenic acids and their derivatives from Stemona japonica by preparative HPLC and evaluation of their anti-AIV (H5N1) activity in vitro. Phytochem Anal 18:213–218
Götz M, Bögri T, Gray AH, Strunz GM (1968) The structure of tuberostemonine. Tetrahedron 24:2631–2643
Greger H (2006) Structural relationships, distribution and biological activities of Stemona alkaloids. Planta Med 72:99–113
Greger H (2012) The diversity of Stemona stilbenoids as a result of storage and fungal infection. J Nat Prod 75:2261–2268
Greger H, Zechner G, Hofer O, Vajrodaya S (1996) Bioactive amides from Glycosmis species. J Nat Prod 59:1163–1168
Greger H, Schinnerl J, Vajrodaya S, Brecker L, Hofer O (2009) Pandanus alkaloids in Stemonaceae: finding of a plausible biogenetic origin of Stemona alkaloids. J Nat Prod 72:1708–1711
Guo A, Jin L, Deng ZW, Cai SQ, Guo SX, Lin WH (2008) New Stemona alkaloids from the roots of Stemona sessilifolia. Chem Biodivers 5:598–605
Guo J, He HP, Li SL, Hua HM, Hao XJ (2010) A new alkaloid from the roots of Stemona tuberosa (Stemonaceae). Acta Bot Yunnanica 32:163–165
Han H, Yang L, Ding Y, Ji G, Zhang T (2016) Pharmacokinetics and metabolism profiles of protostemonine in rat by liquid chromatography combined with electrospray ionization tandem mass spectrometry. J Pharm Biomed Anal 117:266–275
Harada H, Irie H, Masaki N, Osaki K, Uyeo S (1967) The stereochemistry and absolute configuration of stenine and tuberostemonine. Chem Commun. https://doi.org/10.1039/c19670000460
Hartl M, Kiehn M (2004) Chromosome numbers and other karyological data of four Stemona species (Stemonaceae) from Thailand. Blumea 49:457–460
Hitotsuyanagi Y, Hikita M, Oda T, Kakuta D, Fukaya H, Takeya K (2007a) Structures of four new alkaloids from Stemona sessilifolia. Tetrahedron 63:1008–1013
Hitotsuyanagi Y, Hikita M, Nakada K, Fukaya H, Takeya K (2007b) Sessilifoliamide I, a new alkaloid from Stemona sessilifolia. Heterocycles 71:2035–2040
Hitotsuyanagi Y, Takeda E, Fukaya H, Takeya K (2008) Sessilifoliamine A and sessilifoliamide J: new alkaloids from Stemona sessilifolia. Tetrahedron Lett 49:7376–7379
Hitotsuyanagi Y, Uemura G, Takeya K (2010) Sessilifoliamides K and L: new alkaloids from Stemona sessilifolia. Tetrahedron Lett 51:5694–5696
Hitotsuyanagi Y, Hikita M, Uemura G, Fukaya H, Takeya K (2011) Structures of stemoxazolidinones A-F, alkaloids from Stemona sessilifolia. Tetrahedron 67:455–461
Hitotsuyanagi Y, Fukaya H, Takeda E, Matsuda S, Saishu Y, Zhu S, Komatsu K, Takeya K (2013a) Structures of stemona-amine B and stemona-lactams M-R. Tetrahedron 69:6297–6304
Hitotsuyanagi Y, Shigemori G, Fukaya H, Hikita M, Zhu S, Komatsu K, Takeya K (2013b) Stemona-amines C-E, new alkaloids from Stemona tuberosa. Tetrahedron Lett 54:6995–6998
Hitotsuyanagi Y, Sekiya Y, Fukaya H, Park HS, Zhu S, Komatsu K (2016) Stemona-amines F and G, new alkaloids from Stemona tuberosa. Tetrahedron Lett 57:5746–5749
Hofer O, Greger H (2000) Sulfur-containing amides from Glycosmis species (Rutaceae). In: Herz W, Falk H, Kirby GW, Moore RE (eds) Progress in the chemistry of organic natural products. Springer, New York
Hosoya T, Yamasaki F, Nakata A, Rahman A, Kusumawati I, Zaini NC, Morita H (2011) Inhibitors of nitric oxide production from Stemona javanica. Planta Med 77:256–258
Hu JP, Yang DH, Lin WH, Cai SQ (2009) Alkaloids from the roots of Stemona tuberosa. Helv Chim Acta 92:2125–2133
Huang SZ, Kong FD, Ma QY, Guo ZK, Zhou LM, Wang Q, Dai HF, Zhao YX (2016) Nematicidal Stemona alkaloids from Stemona parviflora. J Nat Prod 79:2599–2605
Huber CP, Hall SR, Maslen EN (1968) The crystal structure of oxotuberostemonine. Tetrahedron Lett 4081-4084
Iizuka H, Irie H, Masaki N, Osaki K, Uyeo S (1973) X-ray crystallographic determination of the structure of stemonamine, a new alkaloid from Stemona japonica Miq.: isolation of isostemonamine. J Chem Soc Chem Comm. https://doi.org/10.1039/c39730000125
Inthachub P, Vajrodaya S, Duyfjes BEE (2009) Review of the genus Stichoneuron (Stemonaceae). Edinb J Bot 66:213–228
Inthachub P, Vajrodaya S, Duyfjes BEE (2010) Census of Stemona (Stemonaceae) in Thailand. Blumea 55:143–152
Irie H, Harada H, Ohno K, Mizutani T, Uyeo S (1970a) The structure of the alkaloid protostemonine. J Chem Soc D Chem Comm. https://doi.org/10.1039/c29700000268
Irie H, Masaki N, Ohno K, Osaki K, Taga T, Uyeo S (1970b) The crystal structure of a new alkaloid, stemofoline, from Stemona japonica. Chem Comm 17:1066
Ji ZH, Duyfjes BEE (2000) Stemonaceae. In: Wu ZY, Raven PH (eds) Flora of China, vol 24. Science Press, Beijing
Jiang RW, Hon PM, But PPH, Chung HS, Lin G, Ye WC, Mak TCW (2002) Isolation and stereochemistry of two new alkaloids from Stemona tuberosa. Tetrahedron 58:6705–6712
Jiang RW, Hon PM, Xu YT, Chan YM, Xu HX, Shaw PC, But PPH (2006a) Isolation and chemotaxonomic significance of tuberostemospironine-type alkaloids from Stemona tuberosa. Phytochemistry 67:52–57
Jiang RW, Hon PM, Zhou Y, Chan YM, Xu YT, Xu HX, Greger H, Shaw PC, But PPH (2006b) Alkaloids and chemical diversity of Stemona tuberosa. J Nat Prod 69:749–754
Jiwajinda S, Hirai N, Watanabe K, Santisopasri V, Chuengsamarnyart N, Koshimizu K, Ohigashi H (2001) Occurrence of the insecticidal 16,17-didehydro-16(E)-stemofoline in Stemona collinsae. Phytochemistry 56:693–695
Jung KH, Kil YS, Jung J, Park S, Shin D, Lee K, Seo EK, Bae H (2016) Tuberostemonine N, an active compound isolated from Stemona tuberosa, suppresses cigarette smoke-induced sub-acute lung inflammation in mice. Phytomedicine 23:79–86
Kakuta D, Hitotsuyanagi Y, Matsuura N, Fukaya H, Takeya K (2003) Structures of new alkaloids sessilifoliamides A-D from Stemona sessilifolia. Tetrahedron 59:7779–7786
Kaltenegger E, Brem B, Mereiter K, Kalchhauser H, Kählig H, Hofer O, Vajrodaya S, Greger H (2003) Insecticidal pyrido[1,2-a]azepine alkaloids and related derivatives from Stemona species. Phytochemistry 63:803–816
Ke CQ, He ZS, Yang YP, Ye Y (2003) A novel alkaloid from Stemona parviflora. Chin Chem Lett 14:173–175
Kil YS, Han AR, Seo EK (2014) Tuberostemonin O from the roots of Stemona tuberosa. Bull Korean Chem Soc 35:1891–1893
Kondo H, Satomi M, Odera T (1954) Stemona alkaloids. XVI. Alkaloid from root of Stemona tuberosa. Ann Rept ITSUU Lab 5:99–102
Kongkiatpaiboon S, Schinnerl J, Felsinger S, Keeratinijakal V, Vajrodaya S, Gritsanapan W, Brecker L, Greger H (2011) Structural relationships of Stemona alkaloids: assessment of species-specific accumulation trends for exploiting their biological activities. J Nat Prod 74:1931–1938
Kongkiatpaiboon S, Mikulicic S, Keeratinijakal V, Greger H, Gritsanapan W (2013) HPLC simultaneous analysis for quality assessment of Stemona curtisii roots and determination of their insecticidal activities. Ind Crops Prod 43:648–653
Kostecki K, Engelmeier D, Pacher T, Hofer T, Vajrodaya S, Greger H (2004) Dihydrophenanthrenes and other antifungal stilbenoids from Stemona cf. pierrei. Phytochemistry 65(1):99–106
Koyama H, Oda K (1970) Crystal and molecular structure of stemonine hydrobromide hemihydrate. J Chem Soc B Phys Org. https://doi.org/10.1039/j29700001330
Lai DH, Yang ZD, Xue WW, Sheng J, Shi Y, Yao XJ (2013) Isolation, characterization and acetylcholinesterase inhibitory activity of alkaloids from roots of Stemona sessilifolia. Fitoterapia 89:257–264
Lee KY, Jeong EJ, Sung SH, Kim YC (2016) Stemona alkaloids isolated from Stemona tuberosa roots and their inhibitory activity on lipopolysaccharide-induced nitric oxide production. Rec Nat Prod 10:109–112
Leung PHH, Zhang L, Zuo Z, Lin G (2006) Intestinal absorption of Stemona alkaloids in a CaCo-2 cell model. Planta Med 72:211–216
Li EX, Fu CX (2007) A new synonym of Stemona sessilifolia (Stemonaceae). Acta Phytotax Sin 45:399–402
Li SL, Jiang RW, Hon PM, Cheng L, Xu HX, Greger H, But PPH, Shaw PC (2007a) Quality evaluation of Radix Stemonae through simultaneous quantification of bioactive alkaloids by high-performance liquid chromatography coupled with diode array and evaporative light scattering detectors. Biomed Chromatogr 21:1088–1094
Li ZX, Sturm S, Stuppner H, Schraml E, Moser VA, Siegl V, Pfragner R (2007b) The dichloromethane fraction of Stemona tuberosa Lour inhibits tumor cell growth and induces apoptosis of human medullary thyroid carcinoma cells. Biol Targets Ther 1:455–463
Li YY, Wang YY, Taniguchi T, Kawakami T, Baba T, Ishibashi H, Mukaida N (2010) Identification of stemonamide synthetic intermediates as a novel potent anticancer drug with an apoptosis inducing ability. Int J Cancer 127:474–484
Lim D, Lee E, Jeong E, Jang VP, Kim J (2015) Stemona tuberosa prevented inflammation by suppressing the recruitment and the activation of macrophages in vivo and in vitro. J Ethnopharmacol 160:41–51
Limtrakul P, Siwanon S, Yodkeeree S, Duangrat C (2007) Effect of Stemona curtisii root extract on P-glycoprotein and MRP-1 function in multidrug-resistant cancer cells. Phytomedicine 14:381–389
Lin WH, Fu HZ (1999) Three new alkaloids from the roots of Stemona tuberosa. J Chin Pharm Sci 8:1–7
Lin WH, Yin BP, Tang ZJ, Xu RS, Zhong QX (1990) The structure of parvistemonine. Acta Chim Sin 48:811–814
Lin WH, Xu RS, Zhong QX (1991a) Chemical studies on Stemona alkaloids I. Studies on new alkaloids of Stemona parviflora Wright C.H. Acta Chim Sin 49:927–931
Lin WH, Xu RS, Zhong QX (1991b) Chemical studies on Stemona alkaloids II. Studies on the minor alkaloids of Stemona parviflora Wright C. H. Acta Chim Sin 49:1034–1037
Lin WH, Ye Y, Xu RS (1991c) Studies on new alkaloids of Stemona mairei. Youji Huaxue 11:500–503
Lin WH, Ye Y, Xu RS (1991d) Studies on new alkaloids of Stemona mairei. Chin Chem Lett 2:369–370
Lin WH, Ye Y, Xu RS (1992) Chemical studies on new Stemona alkaloids, IV. Studies on new alkaloids from Stemona tuberosa. J Nat Prod 55:571–576
Lin WH, Cai MS, Ying BP, Feng R (1993a) Studies on the chemical constituents of Croomia japonica Miq. Acta Pharm Sin 28:202–206
Lin WH, Wang L, Qiao L, Cai MS (1993b) Two minor alkaloids from the roots of Stemona tuberosa L. Chin Chem Lett 4:1067–1070
Lin WH, Ma L, Cai MS, Barnes RA (1994) Two minor alkaloids from roots of Stemona tuberosa. Phytochemistry 36:1333–1335
Lin LG, Zhong QX, Cheng TY, Tang CP, Ke CQ, Lin G, Ye Y (2006) Stemoninines from the roots of Stemona tuberosa. J Nat Prod 69:1051–1054
Lin LG, Tang CP, Dien PH, Xu RS, Ye Y (2007a) Cochinchistemonine, a novel skeleton alkaloid from Stemona cochinchinensis. Tetrahedron Lett 48:1559–1561
Lin LG, Dien PH, Tang CP, Ke CQ, Yang XZ, Ye Y (2007b) Alkaloids from the roots of Stemona cochinchinensis. Helv Chim Acta 90:2167–2175
Lin LG, Li KM, Tang CP, Ke CQ, Rudd JA, Lin G, Ye Y (2008a) Antitussive stemoninine alkaloids from the roots of Stemona tuberosa. J Nat Prod 71:1107–1110
Lin LG, Leung HPH, Zhu JY, Tang CP, Ke CQ, Rudd JA, Lin G, Ye Y (2008b) Croomine- and tuberostemonine-type alkaloids from roots of Stemona tuberosa and their antitussive activity. Tetrahedron 64:10155–10161
Lin LG, Bao H, Wang A, Tang CP, Dien PH, Ye Y (2014) Two new N-oxide alkaloids from Stemona cochinchinensis. Molecules 19:20257–20265
Lind RJ, Greenhow DT, Blythe J, Goodchild J, Hirst E, Dunbar SJ, Earley FGP (2002) Cyanotropanes: novel chemistry interacting at the insect nicotinic acetylcholine receptor. The BCPC conference—pest & diseases 2002. vol 1: 145–152. The British Crop Protection Council
Liu XY, Wang FP (2015) Recent advances in the synthesis of Stemona alkaloids. Nat Prod Commun 10:1093–1102
Manohar A (2013) In silico analysis of compounds from Stemona tuberosa as an inhibitor for N1 neuraminidase of H5N1 avian virus. Braz Arch Biol Technol 56:21–25
Mungkornasawakul P, Pyne SG, Jatisatienr A, Supyen D, Lie W, Ung AT, Skelton BW, White AH (2003) Stemocurtisine, the first pyrido[1,2,-a]azapine Stemona alkaloid. J Nat Prod 66:980–982
Mungkornasawakul P, Pyne SG, Jatisatienr A, Supyen D, Jatisatienr C, Lie W, Ung AT, Skelton BW, White AH (2004a) Phytochemical and larvicidal studies on Stemona curtisii: structure of a new pyrido[1,2-a]azepine Stemona alkaloid. J Nat Prod 67:675–677
Mungkornasawakul P, Pyne SG, Jatisatienr A, Lie W, Ung AT, Issakul K, Sawatwanich A, Supyen D, Jatisatienr C (2004b) Phytochemical studies on Stemona burkillii Prain: two new dihydrostemofoline alkaloids. J Nat Prod 67:1740–1743
Mungkornasawakul P, Chaiyong S, Sastraruji T, Jatisatienr A, Jatisatienr C, Pyne SG, Ung AT, Korth J, Lie W (2009) Alkaloids from the roots of Stemona aphylla. J Nat Prod 72:848–851
Mungkornasawakul P, Pyne SG, Willis AC, Jatisatienr A, Phuthsuk D, Lie W (2013) 6-Hydroxy-5,6-seco-stemocurtisine: a novel seco-stemocurtsine-type alkanoid. Phytochem Lett 6:602–605
Noro T, Fukushima S, Ueno A, Miyase T, Iitaka Y, Saiki Y (1979) A new alkaloid, croomine, from Croomia heterosepala Okuyama. Chem Pharm Bull 27:1495–1497
Oginuma K, Horiuchi K, Fukuhara T (2001) Karyomorphology of two genera in Stemonaceae. Acta Phytotax Geobot 52:57–63
Pacher T, Seger C, Engelmeier D, Vajrodaya S, Hofer O, Greger H (2002) Antifungal stilbenoids from Stemona collinsae. J Nat Prod 65:820–827
Phuwapraisirisan P, Poapolathep A, Poapolathep S, Tip-Pyang S (2006) In vivo oxytoxin antagonistic effects of pyrrolizidine alkaloids from Stemona sp. and Asparagus racemosus. ACGC Chem Res Comm 20:17–19
Pilli RA, Rosso GB, de Oliveira MCF (2010) The chemistry of Stemona alkaloids: an update. Nat Prod Rep 27:1908–1937
Pudjiastuti P, Pyne SG, Sugiyanto Lie W (2012) Isolation of tuberospironine A, a novel croomine derivative from Stemona tuberosa. Phytochem Lett 5:358–360
Qian J, Zhan ZJ (2007) Novel alkaloids from Stemona sessilifolia. Helv Chim Acta 90:326–331
Ramli RA, Lie W, Pyne SG (2013) Alkaloids from the roots and leaves of Stichoneuron halabalensis and their acetylcholinesterase inhibitory activities. Nat Prod Commun 8:695–698
Ramli RA, Lie W, Pyne SG (2014) Alkaloids from the roots of Stichoneuron caudatum and their acetylcholinesterase inhibitory activities. J Nat Prod 77:894–901
Ramli RA, Pudjiastuti P, Tjahjandaric TS, Lie W, Rattanajak R, Kamchonwongapaisan S, Pyne SG (2015) Alkaloids from the roots of Stemona javanica (Kunth.) Engl. (Stemonaceae) and their anti-malarial, acetylcholinesterase inhibitory and cytotoxic activities. Phytochem Lett 11:157–162
Rinner B, Siegl V, Pürstner P, Efferth T, Brem B, Greger H, Pfragner R (2004) Activity of novel plant extracts against medullary thyroid carcinoma cells. Anticancer Res 24:495–500
Sakata K, Aoki K, Chang CF, Sakurai A, Tamura S, Murakoshi S (1978) Stemospironine, a new insecticidal alkaloid of Stemona japonica Miq. Isolation, structural determination and activity. Agric Biol Chem 42:457–463
Sakulpanich A, Attrapadung S, Gritsanapan W (2017) Insecticidal activity of Stemona collinsiae root extract against Parasarcophaga ruficornis (Diptera: Sarcophagidae). Acta Trop 173:62–68
Sastraruji T, Jatisatienr A, Pyne SG, Ung AT, Lie W, Williams MC (2005) Phytochemical studies on Stemona plants: isolation of stemofoline alkaloids. J Nat Prod 68:1763–1767
Sastraruji T, Jatisatienr A, Issakul K, Pyne SG, Ung AT, Lie W, Williams MC (2006) Phytochemical studies on Stemona plants: isolation of new tuberostemonine and stemofoline alkaloids. Nat Prod Commun 1:813–818
Sastraruji K, Pyne SG, Ung AT, Mungkornasawakul P, Lie W, Jatisatienr A (2009) Structural revision of stemoburkilline from an E-alkene to a Z-alkene. J Nat Prod 72:316–318
Sastraruji K, Sastraruji T, Pyne SG, Ung AT, Jatisatienr A, Lie W (2010) Semisynthesis and acetylcholinesterase inhibitory activity of stemofoline alkaloids and analogues. J Nat Prod 73:935–941
Sastraruji T, Chaiyong S, Jatisatienr A, Pyne SG, Ung AT, Lie W (2011) Phytochemical studies on Stemona aphylla: isolation of a new stemofoline alkaloid and six new stemofurans. J Nat Prod 74:60–64
Sastraruji K, Sastraruji T, Ung AT, Griffith R, Jatisatienr A, Pyne SG (2012) Synthesis of stemofoline analogues as acetylcholinesterase inhibitors. Tetrahedron 68:7103–7115
Schinnerl J, Kaltenegger E, Pacher T, Vajrodaya S, Hofer O, Greger H (2005) New pyrrolo[1,2-a]azepine type alkaloids from Stemona and Stichoneuron (Stemonaceae). Monatsh Chem 136:1671–1680
Schinnerl J, Brem B, But PPH, Vajrodaya S, Hofer O, Greger H (2007) Pyrrolo- and pyridoazepine alkaloids as chemical markers in Stemona species. Phytochemistry 68:1417–1427
Seger C, Mereiter K, Kaltenegger E, Pacher T, Greger H, Hofer O (2004) Two pyrrolo[1,2-a]azepine type alkaloids from Stemona collinsae Craib: structure elucidations, relationship to asparagamine A, and a new biogenetic concept of their formation. Chem Biodivers 1:265–279
Sekine T, Ikegami F, Fukasawa N, Kashiwagi Y, Aizawa T, Fujii Y, Ruangrungsi N, Murakoshi I (1995) Structure and relative stereochemistry of a new polycyclic alkaloid, asparagamine A, showing anti-oxytocin activity, isolated from Asparagus racemosus. J Chem Soc Perkin Trans I:391–393
Sun H, Dong W, Zhang A, Wang W, Wang X (2012) Ultra-performance liquid-chromatography with tandem mass spectrometry performing pharmacokinetic and biodistribution studies of croomine, neotuberostemonine and tuberostemonine alkaloids absorbed in the rat plasma after oral administration of Stemonae Radix. Fitoterapia 83:1699–1705
Takayama H, Ichikawa T, Kuwajima T, Kitajima M, Seki H, Aimi N, Nonato MG (2000) Structure characterization, biomimetic total synthesis, and optical purity of two new pyrrolidine alkaloids, pandamarilactonine- A and -B, isolated from Pandanus amaryllifolius Roxb. J Am Chem Soc 122:8635–8639
Takayama H, Ichikawa T, Kitajima M, Aimi N, Lopez D, Nonato MG (2001) A new alkaloid, pandanamine; finding of an anticipated biogenetic intermediate in Pandanus amaryllifolius Roxb. Tetrahedron Lett 42:2995–2996
Tang CP, Chen T, Velten R, Jeschke P, Eppinghaus-Kintscher U, Geibel S, Ye Y (2008) Alkaloids from stems and leaves of Stemona japonica and their insecticidal activities. J Nat Prod 71:112–116
Tsai YC, Yu ML, El-Shazly M, Beerhues L, Cheng YB, Chen LC, Hwang TL, Chen HF, Chung YM, Hou MF, Wu YC, Chang FR (2015) Alkaloids from Pandanus amaryllifolius: isolation and their plausible biosynthetic formation. J Nat Prod 78:2346–2354
Umsumarng S, Pintha K, Pitchakarn P, Sastraruji K, Sastraruji T, Ung AT, Jatisatienr A, Pyne SG, Limtrakul P (2013) Inhibition of P-glycoprotein mediated multidrug resistance by stemofoline derivatives. Chem Pharm Bull 61:399–404
Umsumarng S, Pitchakarn P, Sastraruji K, Yodkeeree S, Ung AT, Pyne SG, Limtrakul P (2015) Reversal of human multi-drug resistance leukaemic cells by stemofoline derivatives via inhibition of P-glycoprotein function. Basic Clin Pharmacol Toxicol 116:390–397
Umsumarng S, Pitchakarn P, Yodkeeree S, Punfa W, Mapoung S, Ramli RA, Pyne SG, Limtrakul P (2017) Modulation of P-glycoprotein by Stemona alkaloids in human multidrug resistance leukemic cells and structural relationships. Phytomedicine 34:182–190
Umsumarng S, Mapoung S, Yodkeeree S, Pyne SG, Limtrakul P (2018) A pharmacological strategy using stemofoline for more efficacious chemotherapeutic treatments against human multidrug resistant leukemic cells. Asian Pac J Cancer Prev 19:3533–3543
Wang FP, Chen QH (2014) Stemona alkaloids: biosynthesis, classification, and biogenetic relationships. Nat Prod Commun 9:1809–1822
Wang P, Liu AL, An Z, Li ZH, Du GH, Qin HL (2007a) Novel alkaloids from the roots of Stemona sessilifolia. Chem Biodivers 4:523–530
Wang P, Qin HL, Li ZH, Liu AL, Du GH (2007b) A new alkaloid from Stemona sessilifolia. Chin Chem Lett 18:152–154
Wang YZ, Tang CP, Dien PH, Ye Y (2007c) Alkaloids from the roots of Stemona saxorum. J Nat Prod 70:1356–1359
Wu YX, He HQ, Nie YJ, Ding YH, Sun L, Qian F (2018) Protostemonine effectively attenuates lipopolysaccharide-induced acute lung injury in mice. Acta Pharmacol Sin 39:85–96
Xiang J, Cheng S, Feng TL, Wu Y, Xie W, Zhang M, Xu XH, Zhang CF (2016) Neotuberostemonine attenuates bleomycin-induced pulmonary fibrosis by suppressing the recruitment and activation of macrophages. Internat Immunopharmacol 36:158–164
Xu RS, Lu YJ, Chu JH, Iwashita T, Naoki H, Naya Y, Nakanishi K (1982) Studies on some new Stemona alkaloids. A diagnostically useful 1H NMR line-broadening effect. Tetrahedron 38:2667–2670
Xu RS, Tang ZJ, Feng SC, Yang YP, Lin WH (1991) Studies on bioactive components from Chinese medicinal plants. Mem Inst Oswaldo Cruz, Rio de Janeiro 86(Suppl II):55–59
Xu YT, Hon PM, Jiang RW, Cheng L, Li SH, Chan YP, Xu HX, Shaw PC, But PPH (2006) Antitussive effects of Stemona tuberosa with different chemical profiles. J Ethnopharmacol 108:46–53
Xu YT, Shaw PC, Jiang RW, Hon PM, Chan YM, But PPH (2010) Antitussive and central respiratory depressant effects of Stemona tuberosa. J Ethnopharmacol 128:679–684
Yang XZ, Zhu JY, Tang CP, Ke CQ, Lin G, Cheng TY, Rudd JA, Ye Y (2009) Alkaloids from roots of Stemona sessilifolia and their antitussive activities. Planta Med 75:174–177
Ye Y, Velten RF (2003) Semi-syntheses of new stemofoline derivatives. Tetrahedron Lett 44:7171–7173
Ye Y, Xu RS (1992) Studies on new alkaloids of Stemona japonica. Chin Chem Lett 3:511–514
Ye Y, Qin GW, Xu RS (1994a) Alkaloids from Stemona tuberosa. Phytochemistry 37:1201–1203
Ye Y, Qin GW, Xu RS (1994b) Alkaloids from Stemona japonica. Phytochemistry 37:1205–1208
Ye Y, Qin GW, Xu RS (1994c) Alkaloids of Stemona japonica. J Nat Prod 57:665–669
Yi M, Xia X, Wu HY, Tian HY, Huang C, But PPH, Shaw PC, Jiang RW (2015) Structures and chemotaxonomic significance of Stemona alkaloids from Stemona japonica. Nat Prod Commun 10:2097–2099
Yue Y, Deng AJ, Xu DS, Qin HL (2013) Two new Stemona alkaloids from Stemona tuberosa Lour. J Asian Nat Prod Res 15:145–150
Yue Y, Deng AJ, Li ZH, Liu AL, Ma L, Zhang ZH, Wang XL, Du GH, Qin HL (2014) New Stemona alklaloids from the roots of Stemona tuberosa. Magn Reson Chem 52:719–728
Zhang RR, Tian HY, Wu Y, Sun XH, Zhang JL, Ma ZG, Jiang RW (2014) Isolation and chemotaxonomic significance of stenine- and stemoninine-type alkaloids from the roots of Stemona tuberosa. Chin Chem Lett 25:1252–1255
Zhong Y, Gao Y, Guo QP, Li WM (2010) Two new alkaloids from Stemona tuberosa. Helv Chim Acta 93:133–138
Zhou Y, Jiang RW, Hon PM, Xu YT, Chan YM, Chan TWD, Xu HX, Ding LS, But PPH, Shaw PC (2006) Analyses of Stemona alkaloids in Stemona tuberosa by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 20:1030–1038
Zhou X, Leung PHH, Li N, Ye Y, Zhang L, Zuo Z, Lin G (2009) Oral absorption and antitussive activity of tuberostemonine alkaloids from the roots of Stemona tuberosa. Planta Med 75:575–580
Zou CY, Fu HZ, Lei HM, Li J, Lin WH (1999) New alkaloids from the roots of Stemona japonica Miq. J Chin Pharm Sci 8:185–189
Acknowledgements
Open access funding provided by University of Vienna.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Greger, H. Structural classification and biological activities of Stemona alkaloids. Phytochem Rev 18, 463–493 (2019). https://doi.org/10.1007/s11101-019-09602-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-019-09602-6