Abstract
Betulin, a pentacyclic triterpene and a plant pentacyclic triterpene metabolite, can be found in large quantities in the outer bark of the birches (Betula, Betulaceae). Betulinic acid, obtained by betulin oxidation, is also abundantly present in nature. Both compounds show a wide spectrum of biological and pharmacological properties, such as anti-HIV, anti-inflammatory, and, considered the most important, anti-cancer. Although the specific mechanism of action of betulin against malignant cells is still a subject of detailed research, the activity of betulin acid has been linked to the induction of the intrinsic pathway of apoptosis. As this process occurs with the sparing of non-cancer cells, and the induction of apoptosis can occur under conditions in which standard therapies fail, both substances seem as promising experimental anti-cancer drugs. The aim of this review is to comprehensively summarise the potential of betulin and betulinic acid, both in vitro and in vivo. The discovery, structure, organic synthesis and derivatives forming were shortly described. Also, the potential molecular mechanisms of action and numerous medical applications of betulin and betulinic acid were presented, including previous studies of anti-cancer activity of the compounds, with listed cancer cell types susceptible to therapy.
Similar content being viewed by others
Introduction
Terpenes, interchangeably referred to as isoprenoids or terpenoids, are a group of organic compounds, widely distributed in a variety of plants. Due to their ubiquity and variety in structure and features, they possess a range of physiological and commercial applications (Aldred 2009). The known number of terpenoids isolated from various plants, animals and microbes species is estimated at over 40,000.
Terpenes are biosynthetically-derived units of isopentenyl pyrophosphate (IPP). IPP can be synthesised by plants through the mevalonic acid pathway or the methylerythritol phosphate pathway (Okada 2011). The former reaction takes place in the cytosol, while the latter—in the plastids of plants.
All isoprenoids consist of five-carbon isoprene units and are classified by the number of units involved. The classification, known as biogenetic isoprene rule or C5 rule, was established in Ruzicka (1953).
The group comprising 30-carbon terpenoids is known as triterpenes or triterpenoids. They are biosynthesised by the cyclisation of squalene and are estimated to form at least 50% of all known terpenoids (Rabi and Bishayee 2009; Liby et al. 2007). The most commonly occurring structures of triterpenes are pentacyclic—among which lupane is noteworthy—and tetracyclic triterpenes (Nazaruk and Borzym-Kluczyk 2015). A wide range of basic backbone modifications of triterpenes has been known. In nature, triterpenoids are distributed either in free form, as triterpenic glycosides (many of them termed saponins), as phytosterols or as their precursors (Patlolla and Rao 2012).
Majority of triterpenes, predominantly pentacyclic, are widely distributed in plants—in seeds, roots, stem bark, roots, leaves or the wax-like coating of numerous fruits and herbs, like thyme, mistletoe, wild jujube or lavender (Bishayee et al. 2011; El-Askary et al. 2011; Liu et al. 2016). They also occur naturally in animals and fungi (Silchenko et al. 2012; Ragasa and Cornelio 2013). The ubiquity of triterpenoids in natural environment resulted in conducting various studies in order to explore and use their therapeutic potential. Although triterpenes were considered biologically inactive for a long time, numerous researches in recent years have revealed plenty of their properties, such as anti-cancer, anti-inflammatory, antioxidant, antiviral, hepatoprotective, or cytotoxic (Hordyjewska et al. 2018). However, with several exceptions, pentacyclic triterpenes usually account for less than 0.1% of plant organs dry weight (Jäger et al. 2009).
One of the abovementioned exceptions is the bark of white birch, containing circa 34% of a pentacyclic triterpenoid, betulin (Laszczyk 2009). Canadian scientists also succeeded in obtaining 56% of betulin in the extract of the yellow birch bark (Diouf et al. 2009). Studies have shown that it is also feasible to conduct efficient birch bark extraction, obtaining, respectively, up to 70% and 90% of active compound (Dehelean et al. 2012a, b; Kovalenko et al. 2009).
Betulin
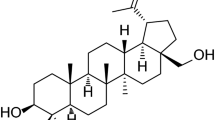
Betulin (BE, lup-20(29)-ene-3β,28-diol, betulinol, betulinic alcohol, Fig. 1a) is a lupane-type compound, characterised by isopropylidene group and five-membered ring (Soica et al. 2012). It can be easily obtained from more than two hundred plants species, although the richest source of BE is the Betulaceae family, especially Betula alba, B. pubescens, B. platyphylla and B. pendula (Dehelean et al. 2012a, b; Lin et al. 2010).
BE occurs in spring as the crystal clusters in the thin-walled, large cells and is responsible for the white colour of the birch bark (Zdzisińska et al. 2010). The amount of BE in the dry weight varies from 10% to even 45%, and this broad range is the consequence of numerous factors, like tree species and age, weather conditions, regional location etc. (Yogeeswari and Sriram 2005; Kuznetsova et al. 2014). BE is also present in the root skin and leaves, although the content is significantly lower when compared to the outer bark (Šiman et al. 2016).
BE can be isolated from the birch bark by means of sublimation or extraction with organic solvents, including acetone, ethanol and chloroform. The amount of BE in the bark extracts varies from 70 to 80% and depends on the birch species (Zdzisińska et al. 2010). The other products of isolation are other terpenes: lupeol, betulinic acid (up to 12%), betulinaldehyde or oleanolic acid (Krasutsky 2006). With its isolation dated to 1788, BE remains one of the first naturally-derived chemical substances to have ever been extracted.
Despite abundant properties of BE, the uttermost limitation in its clinical application is its poor solubility in aqueous media. Nevertheless, an undisputable advantage of betulin is its easiness to form more soluble derivatives (Orchel et al. 2014). Many compounds obtained this way rendered to be highly useful in science (Table 1).
Betulinic acid
One of BE derivatives, a product of its oxidation, is betulinic acid (BA, 3β-hydroxy-lup-20(29)-en-28-oic acid, Fig. 1b). This compound wields a special place among naturally-derived remedies, since outer birch bark, containing BA as an active substance, has been used in folk medicine for centuries (Tsai et al. 2011).
BA, alongside with betulin, is present in the outer bark of many tree species, but its amount in plants is relatively small (Drag-Zalesinska et al. 2009). The exception is a swampy plant, Menyanthes trifoliata, whose underground parts contain significant amounts of free BA (Zdzisińska et al. 2010).
It can also be obtained through extraction from various plant species. By methanolic extraction, BA can be isolated from Quisqualis fructus, the aerial parts of Vietnamese Orthosiphon stamineus, the leaves of Vitex negundo, Combretum quadrangulare and Eucalyptus camaldulensis, stem barks of Tetracentron sinense and Physocarpus intermedium and twigs of Ilex macropoda (Yogeeswari and Sriram 2005),
BA can also be isolated from the ethanolic extracts of different plant species: Tovomita krukovii, Ipomoea pes-caprae, Ancistrocladus heyneanus, Diospyros leucomelas, from the roots of Anemone raddeana and Uapaca nidida, leaves of Doliocarpus schottianus and Syzrgium claviforum and from fruits of Chaenomeles lagenaria (Yogeeswari and Sriram 2005; Zhou et al. 2016).
In terms of BA biosynthesis, two major steps are to be distinguished—lupeol synthesis by 2,3-oxidosqualene cyclisation and, subsequently, oxidation in the C28 position by the enzymes of cytochrome P450 (CYP) (Wu et al. 2017). Several genes encoding the enzymes critical for these reactions have been characterised. Genes responsible for encoding lupeol oxidase have been recently discovered in Arabidopsis thaliana and B. platyphylla bark (Herrera et al. 1998; Zhou et al. 2016). CYP genes encoding C28 oxidase have been found in Vitis vinifera, Catharanthus roseus, Medicago truncatula, and Panax gensing (Fukushima et al. 2011; Han et al. 2011; Huang et al. 2012).
The discovery and characterisation of the abovementioned genes resulted in a breakthrough in the synthesis of BA by genetic engineering methods. By successfully combining the expression of the C28 oxidase from C. roseus and lupeol synthase from A. thaliana, BA was obtained in Saccharomyces cerevisiae from endogenous yeast 2,3-oxidosqualene (Huang et al. 2012; Li and Zhang 2014, 2015).
Several chemical methods of BA synthesis from BE have been provided. Cichewicz et al. established a technique based on two steps: Jones oxidation of the C3 and C28 hydroxyls and then reduction of the resulting compound, C28 betulonic acid, by sodium borohydride (Fig. 2). Other approaches were also provided, among which was a five-step method, where the C28 group in BE was protected and then acetylated of the secondary C3 alcohol. Then, C28 protective group is removed, C28 hydroxyl is oxidised and C3 acetyl group is hydrolysed (Fig. 3) (Kim et al. 1997).
A scheme of five-step synthesis of BA (2) from BE (1). Betulin, as a primary alcohol, was monoprotected as THP ester. Then, the created secondary alcohol underwent acetylation, the THP was successfully removed and, subsequently, carboxylic acid acetate was obtained by Jones oxidation from the primary alcohol acetate. The last step, resulting in formation of BA, was the removal of the acetyl group (Reproduced from Kim et al.)
This BE derivative has been known to scientists for over 100 years, since its identification and first isolation is dated to 1902, but only a few decades ago further interest was attracted to its properties and potential (Retzlaff 1902). Attention was drawn to the results of the study conducted by the group of scientists from the University of Illinois (Pisha et al. 1995).
In that research, BA was described as a potential selective inhibitor of human melanoma cells (Pisha et al. 1995). BA was used against numerous cell lines obtained from human metastatic melanomas: MEL-1, MEL-2, MEL-3 and MEL-4, with IC50 values oscillating between 0.5 and 4.8 μg/ml. Research, consisted of in vitro and in vivo studies, has demonstrated tumour-related cytotoxicity of BA towards melanoma cell lines in mice: MEL-1, a line derived from a lymph node, MEL-2 from pleural fluid, and MEL-4 from a primary skin tumour.
Since this milestone, anti-cancer features of BA has been widely studied in tumour samples, cancer cell lines and animal models. Attention was also drawn to other activities of BA and BE, and researches have been carried out for over two decades to determine the entire spectrum of their growing biological applications.
So far, several reviews combining the current knowledge about betulin or betulin acid have been published (Yogeeswari and Sriram 2005; Fulda 2008, 2009; Ghaffari Moghaddam et al. 2012; Gheorgheosu et al. 2014; Csuk 2014; Król et al. 2015; Rastogi et al. 2015; Zhang et al. 2016; Hordyjewska et al. 2018). However, most of them focus on either BE or BA. In this review, the aim was to collect, describe and compare all the most relevant data of the studies on BA and BE in recent years, from the already mentioned paper by E. Pisha, to the latest results indicating still widening spectrum of their applications. It was also aimed at collecting all the available data regarding the anti-cancer activity of both BE and BA, including mechanisms of action, the in vitro results and translating these results into in vivo action.
Apoptosis
The vast majority of studies held over last decades were aimed to uncover the molecular mechanisms of BA cytotoxic effect on malignant cells. It was soon discovered that this specific activity is resulted by the ability of BA to trigger mitochondrial pathway of apoptosis (Fulda 2008).
Apoptosis, also known as programmed cell death, is a programme of damaged or stressed cells, resulting in a sequence of organised reactions leading to the death of the cell (Green and Kroemer 2004). It may be observed in both physiological and pathological situations. Two major pathways mediating apoptosis can be distinguished: the extrinsic (death receptor) and the intrinsic (mitochondrial) pathway.
Extrinsic pathway
The extrinsic pathway is dependent on the receptor-ligand ligation. The pathway is triggered upon binding of a ‘death ligand’, e.g. CD95-L (FasL, APO-1L) or TRAIL, to its ‘death receptor’, e.g. CD95 (FasR, APO-1) or TRAIL receptor. The binding leads through the intermediate membrane protein, Fas-associated death domain protein (FADD), to forming of the death-inducing signalling complex (DISC), resulting in activation of initiator caspases: caspase-8 and caspase-10. Activated caspases cleave and enable a chain reaction in which effector caspases, including caspase-3, -6, and -7, enter the pathway (Abbas et al. 2015).
Intrinsic pathway
The intrinsic pathway is activated by DNA damage caused by various agents, such as radiation, chemotherapeutic agents etc. and is particularly connected to mitochondrial outer membrane permeabilisation (MOMP). This process, regardless of the morphological type of cell death, is often a decisive point of whether the cell will survive or die. In contrast to the extrinsic pathway, mitochondrial pathway is based mainly on the activity of the Bcl-2 family proteins, which can be divided into three groups: pro-apoptotic, multi-domain proteins (Bax, Bak etc.), with three BH domains; pro-apoptotic, BH3-only proteins (Bad, Bid, Puma, Noxa etc.) showing homology only in the BH3 domain, and anti-apoptotic proteins (e.g. Bcl–XL, Bcl-2, Mcl-1) with four BH domains (Caroppi et al. 2009; Delbridge et al. 2014). The balance between pro-apoptotic and pro-survival proteins is obtained by the activation of BH3-only proteins, that results in mitochondrial membrane permeabilisation and cytochrome c release (Cory and Adams 2002).
These molecules, located in the outer membrane of the mitochondria, under normal conditions are responsible for inhibition of apoptosis. However, when the cell is exposed to stress, one of the proteins—Bax—inhibits the action of Bcl-2 and permeabilises the membrane. As a consequence, numerous compounds are released through the membrane pores, including cytochrome c. As early as in the cytosol, cytochrome c enables the activation of caspase-3 by forming a complex called apoptosome with caspase-9 (Ouyang et al. 2012).
Apoptosis in cancer
Excessive apoptosis is involved in degeneration of various tissues, including muscle tissue or neurons (Fulda and Kroemer 2009). Reversely, disruption of programmed cell death is characteristic for human cancer cells, constituting one of the six most important hallmarks of cancer (Fig. 4) (Hanahan and Weinberg 2011). It is possible considering the feature of cancer cells—the capability to disable the intrinsic apoptosis pathway.
Thus, restoration of a proper mitochondrial pathway with following death of malignant cells was considered a major goal in anti-cancer therapy. However, apoptosis in cancer-altered cells is often severely impaired or even blocked by mutation of genes regulating cell cycle or unbalanced pro- and anti-apoptotic proteins ratio (Laszczyk 2009). For this reason, it is necessary to channel the therapy on various stages of the cell cycle. As a result, it might be possible to avoid miscellaneous blocks, differing depending on the type of cancer.
Since different escape mechanisms were developed by various cancer types, cancer cells often become resistant to conventional therapies (Gimenez-Bonafe et al. 2009). Thus, in most cases, the goal is achieved indirectly, e.g. by triggering a response to DNA damage that activates the mitochondrial apoptotic pathway.
As almost 50% of tumours are characterised by inactivated p53 pathway due to excessive inhibition, reduced activation or acquired mutations, types of treatment focused on the induction of p53-related apoptosis do not produce positive results (Green and Kroemer 2009). Therefore, the goal is to find such anti-cancer agents, that could overcome drug resistance of cancer cells by mechanisms independent of p53, especially if their action is based on the induction of apoptosis. One of those agents is BA (Fulda et al. 1997). However, the role of p53 in BA-induced apoptosis is debatable.
A mechanism of apoptosis independent of p53 was suggested, in which BA and anti-cancer drugs cooperated to induce apoptosis in various tumour cell lines, including mutant p53 cells (Fulda and Debatin 2005). No accumulation of wild-type p53 after BA treatment was detected.
In cells of neuroectodermal tumour or ME20 melanoma cells, apoptosis induced by BA was reported to be p53-independent; meanwhile, expression of p53 was observed in C8161 melanoma cells (Rieber and Rieber 1998; Selzer et al. 2000). However, in various studies examining the effect of BA on mutated cell lines or wild-type p53 lines the difference in sensitivity was not observed (Zuco et al. 2002; Kessler et al. 2007).
Mechanisms of molecular activity
Activity of betulin
The most powerful feature of BE and BA, deciding on their cytotoxicity and antiproliferative potential, is its ability to trigger cell death in malignant cells. Various studies have demonstrated that BE is able to induce apoptosis in numerous cancer cell lines (Ouyang et al. 2012; Zhang et al. 2016). Interestingly, the sensitivity to BE treatment is dependent on cell type—each cancer cell type differs in the patterns of apoptosis-associated proteins expression (Plati et al. 2008).
The study conducted by Li et al. (2010) has proven that cytomorphological features that alter during BE treatment are also characteristic for apoptotic cells. This includes, as listed in the study, cell rounding, membrane blebbing, chromatin condensation, nuclear fragmentation, and formation of apoptotic bodies. This study also proves that BE, unlike BA, did not directly trigger mitochondrial cytochrome c release in isolated mitochondria.
Besides, a study conducted by Potze et al. (2014) demonstrated that inhibition of HeLa cells proliferation was also preceded by characteristic morphological changes, such as cell shrinkage and cariopyknosis, following a 24-h exposure to BE and depending on the dose.
In normal conditions, apoptosis is induced to prevent proliferation of cells suffering from cell cycle disturbances. Properly, cell cycle enables duplication of genetic material and division of the cell (Otto and Sicinski 2017). Cell cycle progression is dependent on the correct occurrence of four phases—G0/G1, S, G2 and M, which is, in turn, controlled by cyclins—a group of regulatory proteins that, in a timely manner, have an influence on cell cycle-dependent kinases (CDKs) (Malumbres and Barbacid 2009).
The activity of CDK-cyclin complexes is precisely controlled: mitotic signals enable their induction and in order to stop CDKs in response to DNA damage, cell cycle checkpoints are activated. In cancer cells, in association with alterations resulting in tumour growth, frequent mutations deregulating CDK-cyclin complexes can be observed. Proliferation is either continued despite inhibitory signals, or the cell re-enters the cycle regardless of the need. Hence, sustaining proliferative signalling and avoiding growth suppressors have been recognised as a part of the characteristic features of human cancer (Fig. 4).
Thus, various researches aim to obtain certain anti-cancer agents that work as a cell cycle re-regulators by inducing cell cycle arrest and enable apoptosis (Ho et al. 2009; Alimova et al. 2009; Liu et al. 2012). One of those agents is BE, although there are hardly any researches examining its influence on cell cycle perturbations.
BE was used in the study, where its role in counteracting cadmium-induced apoptosis in hepatoma cells was investigated. In the study, HepG2 and Hep3B human hepatoma cell lines were used. The results have shown that the influence of BE depends on cell type. Treatment of HepG2 cells induced cell cycle arrest in the late G0/G1 phase and the early S phase, along with a lessening number of cells in the G2/M phase. In turn, after using Hep3B cells, BE has shown a suppressing effect on the G2/M phase. Furthermore, it was suggested in the study that BE might induce a transient reduction of DNA replication with no influence on cycle-regulating genes, p21 and p53 (Oh et al. 2006).
It has been also demonstrated that, at a 10 μM concentration, BE arrests cell cycle of murine melanoma B164A5 cells in S phase. Concurrently, the number of cells in the G0/G1 phase is decreased (Soica et al. 2012).
BE treatment has been also shown to induce apoptosis in vivo in A549 cell line (human lung adenocarcinoma cells) and murine melanoma cells B164A5, in which almost equal quantity of necrotic and apoptotic cells were found after the treatment (Jae et al. 2009; Soica et al. 2012).
In more detailed studies it was indicated that human cancer cells of A549, Jurkat and HeLa lines treated by BE undergo the intrinsic pathway of apoptosis (Mullauer et al. 2009; Li et al. 2010). BE is involved in the sequential activation of caspase-9, caspases 3 and 7, and cleaving of poly(ADP-ribose) polymerase (PARP) (Potze et al. 2014). PARP cleaves to the 85 kDa form, and as PARP is a caspase-3 substrate, this points at caspase-related apoptosis. Since caspase-8 remains inactive, a lack of extrinsic pathway activation has been demonstrated. In turn, caspase-9 has been shown to be initially activated, followed by immediate translocation of proapoptotic proteins—Bax and Bak, and release of cytochrome c and Smac, as a result of mitochondrial membrane potential loss (Li et al. 2010; Potze et al. 2014).
One of the latest studies, presented in 2018 by researchers from Shanghai Jiao Tong University—Zhou et al. (2018) showed a significantly elevated Noxa and Bim mRNA level, while PUMA and Bad remained unchanged after BE treatment. Downregulating Noxa noticeably suppressed the release of cytochrome c and apoptosis in colon cancer cells. Furthermore, the overexpression of Noxa additionally enhanced BE-induced apoptosis.
The results demonstrated that induction of Noxa is essential for the release of cytochrome c as well as for apoptotic response of colon cancer cells treated by BE. As a minor increase in cytochrome c in the cytosolic fraction has been shown after Noxa knockdown, researchers concluded that other mechanisms might also be involved.
Moreover, BE has also been reported not to alter the total expression of mitochondrial pro-apoptotic proteins—Bax and Bcl-2, on both mRNA and protein level (Rzeski et al. 2009).
Betulinic acid activity
BA-induced apoptosis has been described as proceeding with the activation of caspases, mitochondrial permeabilisation and increased reactive oxygen species (ROS) production, but without the involvement of CD95 receptor/ligand or wild-type p53 protein systems (Gheorgheosu et al. 2014; Zhang et al. 2016). The caspase-related procedure was proved by N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (zVAD.fmk), a broad-spectrum caspase inhibitor, that also inhibits BA-related apoptosis (Fulda 2009). However, BA was found to induce cell death in Jurkat cells despite the presence of zvAD.fmk. Meanwhile, PARP cleaving and DNA fragmentation were completely blocked (Kessler et al. 2007). In this regard, the cytotoxic effect of BA was suggested not to be fully dependent on caspases; other cell death pathways are likely to co-participate in its mechanism of action.
There are several mechanisms considered to influence the anti-cancer activity of BA. Among all, the most frequently discussed can be distinguished: already discussed apoptosis of cancer cells via the activation of the mitochondrial pathway of apoptosis; suppressor effect of topoisomerase I and IIα and aminopeptidase N; inhibition of angiogenesis, or activation by phosphorylation of mitogen-activated protein kinase (MAPK) proteins—stress-activated protein/Jun NH2-terminal kinase (SAP/JNK) and p38 (Chowdhury et al. 2002; Tan et al. 2003; Petrovic et al. 2007; Madonna et al. 2012; Luo et al. 2016).
BA has been proven to enhance the level of reactive oxygen species (ROS), which is indirectly involved in triggering cell apoptosis (Shin et al. 2011). It has been indicated that there is a connection between ROS and abovementioned proteins in melanoma cells. Thus, ROS was reported to act upstream of MAPKs in BA signalling pathway (Tan et al. 2003).
Aminopeptidase N, a transmembrane tumour angiogenesis regulator, is highly expressed in newly forming tumour vessels. Thus, the ability of BA to efficiently inhibit aminopeptidise N was widely investigated (Petrovic et al. 2007). In one case, BA was proven to be an angiogenesis inhibitor; hence the researchers concluded that the anti-melanoma effect of BA is due to its efficient aminopeptidase N suppression (Melzig and Bormann 1998). Another study also confirmed the anti-angiogenesis effect of BA, but the reason of that was not its impact on aminopeptidase N, but on mitochondria of endothelial cells (Kwon et al. 2002). Therefore, there is no clear evidence of how significant for cancer cell death the inhibition of aminopeptidase N by BA is.
BA was also shown to inhibit the proliferation of topoisomerases and therefore express anti-proliferative activity (Luo et al. 2016). Topoisomerases, ubiquitous, nucleus-located family of enzymes, participate in DNA fracturing and supercoiling. BA was reported to inhibit these enzymes catalytically. In one study, it was suggested that BA acts through preventing topoisomerase-DNA binding (Wada and Tanaka 2005). Another study, however, pointed out that silencing of topoisomerase I did not affect cell death induced by BA (Ganguly et al. 2007).
However, it is possible that the effect of topoisomerase inhibition may be somehow extinguished by one of the various cytotoxic effects attributed to BA. What is more, several semi-synthetic derivatives of BA were obtained, known to strongly inhibit topoisomerases I and IIα (Abdel Bar et al. 2009). Besides, the analogues were proven to possess better cytotoxicity against colon cancer and breast cancer cell lines that BA itself. Hence, BA, creates a potent scaffold for another powerful topoisomerase inhibitors.
Nuclear factor-κB (NF-κB) is a transcription factor known to be an important part of immune and inflammatory responses, carcinogenesis and also a regulator of stress-induced transcriptional activation (Fulda 2008; Prasad et al. 2010). It has been proven that in melanoma cells, NF-κB pathway enables tumour survival, maintenance, resistance to apoptosis and increased metastatic potential (Madonna et al. 2012).
BA was demonstrated to inhibit activating of NF-κB by different factors, including H2O2, cigarette smoke or TNF. In fact, BA inhibits whole TNF-related cascade of NF-κB activation, including IκKβ (subunit β of the IκB kinase) activation, IκBα phosphorylation-induced degradation, phosphorylation and nuclear translocation of p65, and transcription of NF-κB-reporter genes (Gheorgheosu et al. 2014). In different study, BA was also reported to inhibit NF-κB in colon cancer (Rabi et al. 2008). Contradictory to this, it has been also proven that BA-mediated activation of NF-κB in cancer cell lines effects in apoptosis and, what is more, deprivation of NF-κB by using chemical inhibitors (antioxidant, proteasome inhibitor, IKK inhibitor) results in reduction of apoptotic cell death (Kasperczyk et al. 2005). These opposite results may be explained by the differences in types of cancer cell lines used in these studies. Also, this suggests the context-dependent method of BA-related activation of NF-κB (Fulda and Kroemer 2009).
Moreover, in prostate cancer, BA was proposed to kill cancer cells via a selective process, proteasome-dependent degradation of transcription factors known as specificity proteins 1, 3 and 4 (Chintharlapalli et al. 2007). Degradation of these proteins, overexpressed in many tumours, contributes in the activation of proapoptotic and anti-angiogenic responses.
Another approach of elucidating the molecular mechanism of BA-mediated anti-metastatic activity could also be the inhibition of epithelial-mesenchymal transition (EMT) (Gheorgheosu et al. 2014). EMT is known to be an essential part of the transformation from cancer to metastatic cells and thus is considered as one of the hallmarks of cancer progression. The transition begins with the loss of the apical-basal polarity and the adhesion between adjacent epithelial cells. The cells then detach from the basement membrane, acquire an elongated shape, similar to the mesenchymal cell phenotype, increase their migratory capacity and begin expressing markers specific to mesenchyme, such as vimentin and fibronectin. These altered cells then get into the circulatory system, enabling metastases formation (Fig. 5) (Kalluri and Weinberg 2009).
Demonstration of BA anti-metastatic property by reversing epithelial-mesenchymal transition (EMT). When mesenchyme-type, the cells readily separate from each other, since the apical-basal polarity and cell–cell adhesion are lost, and thus increasingly migrate and invade, creating metastases. Induction of the reversed EMT allows the cells to adhere to the basement membrane, to regain their apical-basal polarity and mutual adhesion, which prevents migration and metastasis
Anti-tumoral activity of BE and BA
The ability to trigger apoptosis in cancer cells is recognised to be one of the mechanisms of BE cytotoxicity and its anti-proliferative activity (Fulda 2008). Among the whole range of properties attributed to BE, anti-cancer potential is considered the most important for the development of medicine. Although in recent decades anti-cancer therapies are being constantly improved, malignancies still show high mortality rate and treatment is burdened with numerous side effects. Thus, developing a kind of powerful, non-toxic anti-cancer agent is now considered a priority.
Starting from the study conducted by Pisha et al. (1995), there is a steady increase in research focusing on gaining an insight into the anti-tumour properties of BE and BA. Their cytotoxicity against malignancies has been extensively studied in vitro—in cancer cell samples, as well as in vivo—in tumour cell lines or mouse xenograft models.
Primarily, based on the first data, BA was described to be selectively toxic against melanoma cells (Pisha et al. 1995). Admittedly, it has been investigated that in the melanoma cell lines BA is preferentially cytotoxic against metastatic over non-metastatic (Rieber and Rieber 1998). Currently, however, it is known that the cytotoxic effect of BA includes a much broader panel of cancer cell lines.
Based on reports published so far, it has been proven that not only BA, but also its precursor—BE, can act as a cytotoxicity inducer towards cancer cells. Both substances can therefore be used in the treatment of lung, stomach or pancreatic cancer. Generally, both substances were significantly cytotoxic during tests, although BA was found to be more effective (Rzeski et al. 2009). Yet, there is a significant difference between cytotoxicity of both compounds, noticed by Rzeski et al. Taking into account cells derived from neuroectodermal malignancies (glioma, neuroblastoma), BE appears to be more cytotoxic than its derivative. Additionally, for reasons unknown, all primary tumour cells, especially ones derived from ovarian carcinoma, remained more sensitive to BE than to BA (Rzeski et al. 2006).
Unfortunately, considering poor solubility of BE in aqueous media, its use as a single agent in cancer treatment appears to be a complicated and difficult solution. Thereby, no results of clinical trials using the anti-tumour potential of BE have been published so far (Laszczyk 2009).
Hence, the attention of scientists had to focus on BA, a close derivative of BE, much better soluble and with equally wide range of features.
BA has been successfully used as a selective inhibitor of tumourigenesis against many different types of carcinomas in various established cell lines and primary tumour samples. Interestingly, BA has been reported to inhibit cancer growth and trigger apoptosis not only in cell cultures, but also in xenograft mouse models. Even in the very first study examining BA influence on melanoma cells, Pisha et al. (1995) confirmed its cytotoxicity both in vitro and in mice. The use of xenograft mouse models in subsequent studies led to the conclusion that the survival time of xenograft mice with ovarian cancer treated with BA was much longer than the mice in the control group (Zuco et al. 2002). Another study, on mouse models as well, revealed that BA could inhibit breast cancer growth and lung metastasis (Zeng et al. 2018). Significantly, subsequent BA studies using xenograft mice proved that it is well absorbed and distributed in the tumour at the highest concentration (Shin et al. 1999). There was no noteworthy decrease in weight or systemic toxicity, regardless of administered doses. Both BA and in its precursor, BE, were proven to lack cytotoxicity (Jäger et al. 2008).
BE and BA against various types of cancer cells
BE and its derivative were reported to exhibit a various range of anti-cancer activity, depending on the type of cancer cells considered. BE showed excessed inhibition of proliferation in SKNAS—cells of human neuroblastoma and reduced in human leukaemia cell line, K-562 and melanoma MEL-2 line (Sarek et al. 2005; Rzeski et al. 2009) (Table 2). In BA, the effect has been best pronounced against human melanoma lines and colorectal adenocarcinoma line, HT-29 (Pisha et al. 1995; Rzeski et al. 2006).
Other studies confirmed that BE and BA were not only effective against SKNAS, but also showed significant activity towards different cell lines of neural tumours. For BE, the IC50 values of 4.6, 7.6 and 7.3 were obtained in TE671 line of rhabdomyosarcoma-medulloblastoma and in neuroblastoma lines GOTO and NB-1, respectively (Hata et al. 2003; Rzeski et al. 2009). BA, in turn, was active towards TE671 cells with the IC50 value of 4.4 μg/ml and also displayed wide ranges of values for other types of brain tumours, with 1.8–17.0 μg/ml for different neuroblastoma cell lines, 3.0–15.0 μg/ml for medulloblastoma, and 1.8–12.0 μg/ml for glioblastoma (Schmidt et al. 1997; Fulda et al. 1999; Woldemichael et al. 2003; Rzeski et al. 2006). It was also indicated that BA influences the proliferation of head and neck squamous cancer, with IC50 value of 8.0 towards SCC9 and SCC25 lines (Thurnher et al. 2003).
Both compounds were also found to be effective against FTC238 cell line of thyroid cancer, inhibiting proliferation by half at 3.0 μg/ml for BE and 2.1 μg/ml for BA (Rzeski et al. 2006, 2009).
Anti-cancer potential of BE and its derivative was also examined towards breast cancer, a highly metastatic, most common female cancer in the world. Metastasis poses a high risk of cancer-related death, and the key metastatic factors are proliferation, migration and invasion of breast cancer cells (Weigelt et al. 2005). Hence, investigating whether BE and BA inhibit migration of breast cancer cell has become a priority to implement this substance for treatment. Both triterpenoids elicited a significant effect on T47D and MCF-7 cell lines, although different studies report different IC50 values. The activity against T47D was evaluated for BE, with the results of IC50 = 2.3 and 32.4 μg/ml, and with 1.1 μg/ml for BA (Rzeski et al. 2006, 2009; Boryczka et al. 2013). Significantly closer values were reported for MCF-7, with IC50 for BA = 8.9–12.3 μg/ml and BE = 3.6–10.3 μg/ml (Dehelean et al. 2012a, b; Gauthier et al. 2006, 2009; Li et al. 2010; Park et al. 2014; Şoica et al. 2012; Tiwari et al. 2014).
Furthermore, other studies also reported that BE and BA elicit anti-tumour activity towards small and non-small cell lung carcinomas. According to the statistics provided by the International Agency for Research on Cancer, lung cancer is the most frequently diagnosed cancer in the last decade and the leading cause of cancer-related deaths (Ferlay et al. 2015). Applied against lung cancer cell lines, BE showed IC50 values of 1.7, 3.3, 8.9, 14.8 μg/ml for A549 line, 28.1 μg/ml for H460 line and > 20.0 μg/ml for Lu1 line (Hwang et al. 2003; Gauthier et al. 2006, 2009; Jae et al. 2009; Rzeski et al. 2009; Li et al. 2010). BA was found to have slightly lower IC50 values than BE towards POGB, POGB/DX (3.2–4.2 μg/ml), H460 (1.5 μg/ml) and A549 (1.96 μg/ml) lines (Zuco et al. 2002; Rzeski et al. 2006).
BE and BA have also been evaluated in vitro for its anti-cancer potential towards pancreatic cancer, one of the most lethal malignant neoplasms in the world. Versus EPP85-181P cell line, BE inhibited proliferation by 50% at the concentration of 9.3 μg/ml and IC50 values for BA were 6.9 to 10.0 μg/ml (BxPC3) and 11.4 μg/ml (PANC-1) (Drag et al. 2009; Gao et al. 2011).
Cytotoxic and anti-proliferative effect of BE and its derivative have also been confirmed against other malignancies within the human digestive system. BE exhibited antiproliferative activity towards hepatoma HepG2 cells (IC50 = 10.1 μg/ml), EPG85-257P line of gastric adenocarcinoma (IC50 = 8.3 μg/ml), colon cancer Col2 line (IC50 = > 20.0 μg/ml) and different lines (HT-29 line, IC50 = 1.9 μg/ml; DLD-1 line, IC50 = 2.9 μg/ml; SW707 line, IC50 = 22.9 μg/ml) of colorectal cancer cells (Hwang et al. 2003; Gauthier et al. 2006, 2009; Drag et al. 2009; Rzeski et al. 2009; Li et al. 2010; Boryczka et al. 2013). The best results were displayed towards colorectal adenocarcinoma lines of HT-29 and DLD-1, with relatively low IC50 value. Slightly higher IC50 values have been reported in the studies of BA against gastric and colorectal adenocarcinomas. The concentration for IC50 values has been estimated to 12.99 μg/ml against gastric cancer cell line, AGS, and 1.2 μg/ml against HT-29 (Rzeski et al. 2006; Yang et al. 2010).
Over the years, BE and BA have been widely evaluated for their anti-cancer potential in the treatment of genital neoplasia. The results showed that both compounds had elicited significant anti-proliferative potential towards ovarian, cervix and prostate carcinomas.
In ovarian carcinoma, BA has been found to be significantly more potent than BE. Applied to A2780 cell line, BA inhibited proliferation by half at 1.8–4.5 μg/ml, while the IC50 value of BE was > 20.0 μg/ml (Zuco et al. 2002; Prakash Chaturvedula et al. 2003).
BA was also found to inhibit proliferation of malignant cells in other ovarian cancer lines: OVCAR-5, IGROV-1 (IC50 = 1.8–4.5 μg/ml) and HPOC line (IC50 = 2.5 μg/ml), prostate cancer lines DU145 (IC50 = 6.0 μg/ml) and LNCaP (IC50 = 0.5–2.3 μg/ml), and two different cervix carcinoma cell lines: A431 (IC50 = 1.8 μg/ml) and SiHa (IC50 = 11.8 μg/ml) (Zuco et al. 2002; Rzeski et al. 2006; Chintharlapalli et al. 2007; Kessler et al. 2007; Ganguly et al. 2007).
BE was reported to inhibit HeLa cells proliferation by 81.39% at the concentration of 13.28 μg/ml. It was also found that its IC50 values significantly vary over time: 32.8 μg/ml after 24 h, 25.3 μg/ml after 48 h and 15.2 μg/ml after 72 h (Li et al. 2010; Wang et al. 2012).
A panel of human melanoma cell lines was also pronounced to be susceptible to the antiproliferative and cytotoxic activity of BA and BE. The results for BA were comparable regardless of the cell line, with IC50 value of 1.1–4.6 μg/ml for MEL lines (MEL-1, -2, -3, -3) and 1.5–1.6 μg/ml for Me665/2/60 and Me665/2/21 (Pisha et al. 1995; Zuco et al. 2002). Importantly, a higher BA activity was observed for melanoma cells at lower pH conditions (Cichewicz and Kouzi 2004).
BE exhibited similar results towards G361 and SK-MEL-28 lines (5.5 and 7.2 μg/ml, respectively), and significantly worse against MEL-2 (> 20.0 and > 111.0 μg/ml, depending on the study) (Kim et al. 1998; Hata et al. 2003; Sarek et al. 2005).
Haematological malignancies have also been evaluated for susceptibility to BE and BA, with both showing the relatively strong activity versus Jurkat E.61, IC50 = 3.0 μg/ml for BE and 3.15μg/ml for BA (Rzeski et al. 2006, 2009). BE achieved comparable results against U937 and HL60 lines, IC50 = 6.3 and 6.5 μg/ml, respectively, and a worse outcome versus multiple myeloma RPMI 8226 line (IC50 = 20.0) (Hata et al. 2003; Rzeski et al. 2009). Similar results were also obtained by BA against RPMI 8226, U937 and K-562 with respective IC50 values of 1.96, 4.4 and 4.3 μg/ml, although another study suggested significantly different value for K-562–12.5 μg/ml (Hata et al. 2003; Raghuvar Gopal et al. 2005; Rzeski et al. 2006).
Differences were also noticed in the studies on BE impact on cell lines K-562 and CEM. In some of the studies, BE was considered to be completely inactive, while others confirmed its remarkable anti-proliferative activity (Sarek et al. 2005; Yang et al. 2010; Urban et al. 2012; Boryczka et al. 2013). The reason for these variances may be a different source of BE or differences in the method of its extraction.
BE and BA in combination with other compounds
What is significant, both discussed compounds could be successfully combined with other cytotoxic forms of cancer treatment. Favourable results were obtained by combining BA and, respectively, vincristine, 5-fluorouracil, doxorubicin, etoposide, actinomycin D, mithramycin A, cisplatin, taxol, oxaliplatin, or irinotecan, as well as ionizing radiation (Sawada et al. 2004; Jung et al. 2007; Gao et al. 2011; Bache et al. 2011; Zhao et al. 2012; Csuk 2014). In turn, a combination of BE and sorafenib, a kinase inhibitor effective against various carcinomas, enhanced the induction of apoptosis in different non-small cell lung cancer lines (Kutkowska et al. 2017).
Scientists combined the anti-cancer potential of these agents during in vivo analysis of malignant metastatic melanoma model. The outcome revealed significantly suppressed lung metastasis in mice receiving the combination of vincristine and BA. Meanwhile, in the control group, treated with vincristine alone, the results were far worse. Therefore, it was assumed that BA may be used as an enhancer during chemotherapeutic malignant melanoma treatment.
Furthermore, BA augments cytotoxicity against cancer cell lines not only in combination with chemotherapeutics, but also with other compounds, among which thalidomide, ginsenoside Rh2, or epithelial growth factor receptor tyrosine kinase inhibitor PD153035 can be distinguished (Pandey et al. 2010; Li et al. 2011). The results indicated that, in comparison to normal cells, malignant cells were characterised by much lower resistance to BE and BA, regardless of their origin. Hence, the conclusion is, the cytotoxicity of these compounds is clearly selective (Drag et al. 2009). Similarly, while exposing cancer cells and non-cancer cell lines to hypothermia, the former were observed to be more sensitive to BA, while the sensitivity of the latter remained unchanged (Wachsberger et al. 2002).
Meanwhile, in another study, BE has been indicated to protect renal cells during cisplatin treatment. Cisplatin is a compound broadly used as a chemotherapeutic agent against various types of malignancies, including testicular, ovarian, cervical, head and neck, bladder or small cell lung carcinomas (Florea and Büsselberg 2011). Yet, its nephrotoxicity was an important limitation in its therapeutic use (Miller et al. 2010).
Researchers from the Republic of Korea, H. So et al. examined the bark of Japanese white birch (B. platyphylla var. japonica), BE-enriched plant used in traditional Chinese medicine for kidney diseases treatment. The study evaluated the effect of BE isolated from Japanese white birch bark on cisplatin-induced kidney damage. BE reduced the damage to 80% of the control value from the 5 μM concentration. This proved its nephroprotective properties after cisplatin therapy (So et al. 2018).
Other biological activity of BE and BA
BE and BA, apart from their cytotoxicity, have been associated with many other beneficial biological properties. This includes anti-viral (inhibition of human immunodeficiency virus, ECHO-6 virus and HSV-1), antibacterial, anti-malarial, anti-allergic, anti-angiogenic, anti-inflammatory, anti-fibrotic, anticonvulsant and hepatoprotective activity.
Anti-viral activity of BE and BA was widely tested for various types of viruses. Theo et al. has proven that BA from the stem bark of Peltophorum africanum possesses inhibitory activity against certain types of human immunodeficiency virus: HIV-1NL4-3 and HIV-1JRCSF (X4-HIV-1 and R5-HIV-1, respectively) with IC50 value estimated at 0.04 µg/ml (Theo et al. 2009).
In the research conducted by Ryu et al. BA was obtained from chloroform extract of the whole herb of Prunella vulgaris (Ryu et al. 1992). Its anti-Herpes simplex virus type 1 activity was indicated a significant reduction of the cytopathic effect of HSV-1-infected cells occurred when applied BA. The anti-HSV1 activity was estimated at EC50 of 30 μg/ml.
BE extracted from aerial parts of E. denticulate was also proven to show anti-HSV-1 activity, with EC50 value of approximately 84.37 μg/ml (Shamsabadipour et al. 2013).
In turn, Pavlova et al. demonstrated a coherence in the anti-viral activity of BE and BA, indicating that both compounds, alongside betulonic acid, display activity against ECHO-6 virus (Pavlova et al. 2003).
Numerous studies have been conducted to examine the antimicrobial activity of BA and BE, although the results are conflicting. Isolation of BA from Caesalpinia paraguariensis indicated that no BA activity towards E. coli, B. subtilis, S. aureus, and C. albicans was exhibited with minimum inhibitory concentrations (MICs) above 128 μg/ml (Cichewicz and Kouzi 2004). Moreover, in a subsequent, similar study, determination of MICs also revealed lack of BA antibacterial properties (Fontanay et al. 2008). However, another study confirmed the inhibitory activity of BA isolated from Forsythia suspensa VAHL towards the urease enzyme of H. pylori (Shin et al. 2009). Despite these contradictions, some derivatives of BE were reported to exhibit antibacterial activity, which seems like a promise for future applications (Chue et al. 2011; Haque et al. 2014).
BA were widely investigated for anti-malarial activity in vitro and in vivo, e.g. after isolation from the ethanol extract of the root bark of Uapaca nitida (Steele et al. 1999). BA was described as anti-plasmodial, with IC50 values of 19.6 μg/ml against chloroquine-resistant (K1) and 25.9 μg/ml against chloroquine-sensitive (T9-96) Plasmodium falciparum. During in vivo study of murine malaria model, BA top dosage of 250 mg/kg/day was found ineffective in parasitaemia reduction.
In the study conducted by do Carmo et al. the cytotoxicity against Plasmodium berghei and P. falciparum was assessed in vitro. The results elucidated that pentacyclic triterpenes, with betulin and betulinic acid among them, showed anti-plasmodial effect with IC50 values varying from 5.6 to 80.30 μM (do Carmo et al. 2015).
Among the derivatives of lupane, ursane and oleanane-type triterpenes, BE and BA were reported to exhibit the highest anti-inflammatory activity towards murine ear oedema and skin inflammation induced by various compounds, including mezerein (Alakurtti et al. 2006). However, another study suggested no activity of both lupane derivatives against xylene, resiniferatoxin and arachidonic acid-induced inflammation (Huguet et al. 2000).
In the study by Zdzisińska et al. (2003) BE and BA were presented as potent influencers on cytokine production and, consequently, the modulation of an inflammatory response.
Viji et al. (2011) concluded that BA deters the inflammatory responses in LPS-stimulated human peripheral blood mononuclear cells, by modulations of ERK, Akt and NF-κB.
BA was also indicated to exhibit a potent response against hepatic fibrosis, during both in vitro and in vivo treatment (Wan et al. 2012). Thioacetamide-induced hepatic fibrosis was treated with injections of BA following thioacetamide injections, repeated for 6 to 8 weeks. Evidence provided by the results of the study shown significant anti-fibrotic activity of BA by affecting the signaling pathway of Toll-like receptor 4, myeloid differentiation factor 88 and NF-κB.
A noteworthy feature of BE is the induction of an anticonvulsant effect. It has been demonstrated in vitro and in mice that BE binds to the receptors of gamma-aminobutyric acid–GABA, an inhibitory neurotransmitter of the great importance in the nervous system. Blocking its action results in convulsions, and long-term and significant deficiency of this transmitter leads to death.
BE, by binding to GABA receptors, works agonistically to this neurotransmitter. Hence, it blocks GABA receptors before they are activated by a GABAA antagonist, bicuculline. By the administration of BE, the action of this convulsive factor can be suppressed. BA, unlike BE, does not exhibit such properties (Muceniece et al. 2008).
Remarkable is also the antinociceptive effect of BE—in research on rodents, it is more active than aspirin or paracetamol (de Souza et al. 2007).
A study of hepatoprotective activity of BE and BA was conducted by Shikov et al. (2011). Although the data are not clinically relevant due to the lack of a control group in the study, the results remain interesting. Patients with serologically confirmed chronic hepatitis C (CHC) were given daily 160 mg of birch bark extract containing 75% BE and 3.5% BA. After 12 weeks of treatment, the rate of alanine aminotransferase (ALT) normalisation was investigated. In 54.0% of treated patients, the ALT level was decreased and normalised, and HCV RNA was reduced in 43.2%, which is a hope for the use of BE and BA in the future clinical treatment of CHC.
Derivatives of BE and BA
Numerous researches were conducted to obtain various derivatives of BE and BA. The BA ring skeleton serves as a scaffold for numerous promising modifications (Kumar et al. 2008). BA is available for modifications at various positions, including C2, C20 or C28 (Kim et al. 1998; Dominguez-Carmona et al. 2010) (Table 1). C20 modifications do not improve the cytotoxicity in numerous cell lines, but modifying C3 and C28 positions achieved favourable results (Kim et al. 2001).
Both BE and BA derivatives are known to exhibit the anti-cancer, anti-leishmanial and anti-HIV activity (Sousa et al. 2014; Tang et al. 2014; Zhuo et al. 2018). However, although they seem as promising agents in various form of cancer therapy, the molecular mechanism of action and the targets have not yet been acknowledged (Zhang et al. 2015).
What is more, BE derivatives were proven to hold anti-inflammatory activity by selective inhibition of the inducible NOS in a post-transcriptional manner, alongside with nitric oxide production (Laavola et al. 2016). Some analogues also display anti-plasmodial, antibacterial and anti-HPV type 11 activity (Kazakova et al. 2010; Chue et al. 2011; Banzouzi et al. 2015).
The structure of BA derivatives was used for obtaining drugs inhibiting HIV-1 entry, especially proteases and reverse transcriptase (de Sa et al. 2009; Gautam and Jachak 2009). Moreover, BA derivative named 20,29-dihydro-BA was found to possess better anti-angiogenic activity than its analogue (Fulda and Kroemer 2009).
To conclude, the future use of BE and BA derivatives in therapy will mainly be based on identifying the mechanisms of their molecular action. The faster and more detailed they are explained, the greater the chance of using derivatives in clinical treatment.
Concluding remarks
A naturally occurring triterpenoid, BE, and one of its derivatives, BA, exhibit significant biological potential, due to their ubiquitousness and variety of properties. They are considered to counteract inflammations, allergies, and to be effective versus a wide range of microbes, viruses and, above all, malignancies—as potent inhibitors of tumour proliferation and angiogenesis.
The mechanism of anti-tumour activity of these compounds is based on the induction of apoptosis through the mitochondrial pathway, although the molecular basis of this process is still a contentious issue and is the object of numerous studies. In addition to the effects achieved alone, BE and BA have also been proved to be highly effective in combination with various substances, those used in cancer therapy and more. Positive results were obtained not only in combination with conventional chemo- or radiotherapeutics, but also with other substances, such as thalidomide.
Additionally, an advantage of BA is that despite its limited solubility in aqueous media, it is capable of create a variety of derivatives with many beneficial properties.
Despite modern cancer treatment system is still being improved, the mortality rate among oncological patients remains high, and the agents lack selectivity to malignant cells with sparing non-affected cells. Hence, anti-tumour properties displayed by BE and BA seem to be a hope and a promise for future oncological therapies. Moreover, the ubiquity of BE and its derivative in the natural environment, their high therapeutic potential and beneficial results of researches make the use of these substances in future treatment practice highly probable.
References
Abbas AK, Lichtman AHH, Pillai S (2015) Basic immunology: functions and disorders of the immune system. Elsevier Health Sciences, New York
Abdel Bar FM, Khanfar MA, Elnagar AY et al (2009) Rational design and semisynthesis of betulinic acid analogues as potent topoisomerase inhibitors. J Nat Prod 72:1643–1650. https://doi.org/10.1021/np900312u
Alakurtti S, Mäkelä T, Koskimies S, Yli-Kauhaluoma J (2006) Pharmacological properties of the ubiquitous natural product betulin. Eur J Pharm Sci 29:1–13. https://doi.org/10.1016/j.ejps.2006.04.006
Aldred EM (2009) Pharmacology: a handbook for complementary healthcare professionals, Section 4: Plant pharmacology, Terpenes. Churchill Livingstone/Elsevier, Edinburgh, New York, pp 167–174
Alimova IN, Liu B, Fan Z et al (2009) Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle 8:909–915. https://doi.org/10.4161/cc.8.6.7933
Bache M, Zschornak MP, Passin S et al (2011) Increased betulinic acid induced cytotoxicity and radiosensitivity in glioma cells under hypoxic conditions. Radiat Oncol 6:111. https://doi.org/10.1186/1748-717X-6-111
Banzouzi JT, Soh PN, Ramos S et al (2015) Samvisterin, a new natural antiplasmodial betulin derivative from Uapaca paludosa (Euphorbiaceae). J Ethnopharmacol 173:100–104. https://doi.org/10.1016/j.jep.2015.07.023
Bishayee A, Ahmed S, Brankov N, Perloff M (2011) Triterpenoids as potential agents for the chemoprevention and therapy of breast cancer. Front Biosci 16:980. https://doi.org/10.2741/3730
Boryczka S, Bebenek E, Wietrzyk J et al (2013) Synthesis, structure and cytotoxic activity of new acetylenic derivatives of betulin. Molecules 18:4526–4543. https://doi.org/10.3390/molecules18044526
Caroppi P, Sinibaldi F, Fiorucci L, Santucci R (2009) Apoptosis and human diseases: mitochondrion damage and lethal role of released cytochrome c as proapoptotic protein. Curr Med Chem 16:4058–4065. https://doi.org/10.2174/092986709789378206
Chintharlapalli S, Papineni S, Ramaiah SK, Safe S (2007) Betulinic acid inhibits prostate cancer growth through inhibition of specificity protein transcription factors. Cancer Res 67:2816–2823. https://doi.org/10.1158/0008-5472.CAN-06-3735
Chowdhury AR, Mandal S, Mittra B et al (2002) Betulinic acid, a potent inhibitor of eukaryotic topoisomerase I: identification of the inhibitory step, the major functional group responsible and development of more potent derivatives. Med Sci Monit 8:254–265
Chue K, Chang M, Ten LN (2011) Synthesis and antibacterial activity of betulin esters. Chem Nat Compd 47:583–586
Cichewicz RH, Kouzi SA (2004) Chemistry, biological activity, and chemotherapeutic potential of betulinic acid for the prevention and treatment of cancer and HIV infection. Med Res Rev 24:90–114. https://doi.org/10.1002/med.10053
Cory S, Adams JM (2002) The BCL2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2:647–656. https://doi.org/10.1038/nrc883
Csuk R (2014) Betulinic acid and its derivatives: a patent review (2008–2013). Expert Opin Ther Pat 24:913–923. https://doi.org/10.1517/13543776.2014.927441
de Sa MS, Costa JFO, Krettli AU et al (2009) Antimalarial activity of betulinic acid and derivatives in vitro against Plasmodium falciparum and in vivo in P. berghei-infected mice. Parasitol Res 105:275–279. https://doi.org/10.1007/s00436-009-1394-0
de Souza MT, de Campos Buzzi F, Cechinel Filho V et al (2007) Phytochemical and antinociceptive properties of Matayba elaeagnoides Radlk. barks. Z Naturforsch C 62:550–554
Dehelean CA, Feflea S, Molnar J et al (2012a) Betulin as an antitumor agent tested in vitro on A431, HeLa and MCF7, and as an angiogenic inhibitor in vivo in the CAM assay. Nat Prod Commun 7:981–985
Dehelean CA, Şoica C, Ledeţi I et al (2012b) Study of the betulin enriched birch bark extracts effects on human carcinoma cells and ear inflammation. Chem Cent J 6:137. https://doi.org/10.1186/1752-153X-6-137
Delbridge ARD, Grabow S, Bouillet P et al (2014) Functional antagonism between pro-apoptotic BIM and anti-apoptotic BCL–XL in MYC-induced lymphomagenesis. Oncogene 34:1872
Diouf N, Stevanovic T, Boutin Y (2009) The effect of extraction process on polyphenol content, triterpene composition and bioactivity of yellow birch (Betula alleghaniensis Britton) extracts. Ind Crops Prod 30:297–303
do Carmo DFM, Amaral ACF, Machado M et al (2015) Evaluation of Antiplasmodial activity of extracts and constituents from Ampelozizyphus amazonicus. Pharmacogn Mag 11:S244–S250. https://doi.org/10.4103/0973-1296.166071
Dominguez-Carmona DB, Escalante-Erosa F, Garcia-Sosa K et al (2010) Antiprotozoal activity of betulinic acid derivatives. Phytomedicine 17:379–382. https://doi.org/10.1016/j.phymed.2009.08.002
Drag M, Surowiak P, Drag Zalesinska M et al (2009) Comparision of the cytotoxic effects of birch bark extract, betulin and betulinic acid towards human gastric carcinoma and pancreatic carcinoma drug-sensitive and drug-resistant cell lines. Molecules 14:1639–1651. https://doi.org/10.3390/molecules14041639
Drag-Zalesinska M, Kulbacka J, Saczko J et al (2009) Esters of betulin and betulinic acid with amino acids have improved water solubility and are selectively cytotoxic toward cancer cells. Bioorganic Med Chem Lett 19:4814–4817. https://doi.org/10.1016/j.bmcl.2009.06.046
El-Askary H, El-Olemy MM, Salama M et al (2011) Bioguided isolation of pentacyclic triterpenes from the leaves of Alstonia scholaris (Linn.) R. Br. growing in Egypt. Nat Prod Res 26:1755–1758
Ferlay J, Soerjomataram I, Dikshit R et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–E386. https://doi.org/10.1002/ijc.29210
Florea A-M, Büsselberg D (2011) Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers (Basel) 3:1351–1371
Fontanay S, Grare M, Mayer J et al (2008) Ursolic, oleanolic and betulinic acids: antibacterial spectra and selectivity indexes. J Ethnopharmacol 120:272–276. https://doi.org/10.1016/j.jep.2008.09.001
Fukushima EO, Seki H, Ohyama K et al (2011) CYP716A subfamily members are multifunctional oxidases in triterpenoid biosynthesis. Plant Cell Physiol 52:2050–2061. https://doi.org/10.1093/pcp/pcr146
Fulda S (2008) Betulinic acid for cancer treatment and prevention. Int J Mol Sci 9:1096–1107. https://doi.org/10.3390/ijms9061096
Fulda S (2009) Betulinic acid: a natural product with anticancer activity. Mol Nutr Food Res 53:140–146. https://doi.org/10.1002/mnfr.200700491
Fulda S, Debatin K (2005) Sensitization for anticancer drug-induced apoptosis by betulinic acid. Neoplasia 7:162–170. https://doi.org/10.1593/neo.04442
Fulda S, Kroemer G (2009) Targeting mitochondrial apoptosis by betulinic acid in human cancers. Drug Discov Today 14:885–890. https://doi.org/10.1016/j.drudis.2009.05.015
Fulda S, Friesen C, Los M et al (1997) Betulinic acid triggers CD95 (APO-1/Fas)- and p53-independent apoptosis via activation of caspases in neuroectodermal tumors. Cancer Res 57:4956–4964
Fulda S, Jeremias I, Steiner HH et al (1999) Betulinic acid: a new cytotoxic agent against malignant brain-tumor cells. Int J Cancer 82:435–441. https://doi.org/10.1002/(SICI)1097-0215(19990730)82:3%3c435:AID-IJC18%3e3.0.CO;2-1
Ganguly A, Das B, Roy A et al (2007) Betulinic acid, a catalytic inhibitor of topoisomerase I, inhibits reactive oxygen species-mediated apoptotic topoisomerase I-DNA cleavable complex formation in prostate cancer cells but does not affect the process of cell death. Cancer Res 67:11848–11858. https://doi.org/10.1158/0008-5472.CAN-07-1615
Gao Y, Jia Z, Kong X et al (2011) Combining betulinic acid and mithramycin a effectively suppresses pancreatic cancer by inhibiting proliferation, invasion, and angiogenesis. Cancer Res 71:5182–5193. https://doi.org/10.1158/0008-5472.CAN-10-2016
Gautam R, Jachak SM (2009) Recent developments in anti-inflammatory natural products. Med Res Rev 29:767–820. https://doi.org/10.1002/med.20156
Gauthier C, Legault J, Lebrun M et al (2006) Glycosidation of lupane-type triterpenoids as potent in vitro cytotoxic agents. Bioorg Med Chem 14:6713–6725. https://doi.org/10.1016/j.bmc.2006.05.075
Gauthier C, Legault J, Lavoie S et al (2009) Synthesis and cytotoxicity of bidesmosidic betulin and betulinic acid saponins. J Nat Prod 72:72–81. https://doi.org/10.1021/np800579x
Gheorgheosu D, Duicu O, Dehelean C et al (2014) Betulinic acid as a potent and complex antitumor phytochemical: a minireview. Anticancer Agents Med Chem 14:936–945. https://doi.org/10.2174/1871520614666140223192148
Gimenez-Bonafe P, Tortosa A, Perez-Tomas R (2009) Overcoming drug resistance by enhancing apoptosis of tumor cells. Curr Cancer Drug Targets 9:320–340. https://doi.org/10.2174/156800909788166600
Green DR, Kroemer G (2004) The pathophysiology of mitochondrial cell death. Science 305:626–629. https://doi.org/10.1126/science.1099320
Green DR, Kroemer G (2009) Cytoplasmic functions of the tumour suppressor p53. Nature 458:1127–1130. https://doi.org/10.1038/nature07986
Han J-Y, Kim H-J, Kwon Y-S, Choi Y-E (2011) The Cyt P450 enzyme CYP716A47 catalyzes the formation of protopanaxadiol from dammarenediol-II during ginsenoside biosynthesis in Panax ginseng. Plant Cell Physiol 52:2062–2073. https://doi.org/10.1093/pcp/pcr150
Hanahan D, Weinberg RA (2011) Hallmarks of sation. Cell 144:646–674. https://doi.org/10.1016/j.cell.2011.02.013
Haque S, Nawrot DA, Alakurtti S et al (2014) Screening and characterisation of antimicrobial properties of semisynthetic betulin derivatives. PLoS ONE 9:e102696
Hata K, Hori K, Ogasawara H, Takahashi S (2003) Anti-leukemia activities of Lup-28-al-20(29)-en-3-one, a lupane triterpene. Toxicol Lett 143:1–7. https://doi.org/10.1016/S0378-4274(03)00092-4
Herrera JB, Bartel B, Wilson WK, Matsuda SP (1998) Cloning and characterization of the Arabidopsis thaliana lupeol synthase gene. Phytochemistry 49:1905–1911
Ho K, Saiful L, Ismail N, Ismail M (2009) Apoptosis and cell cycle arrest of human colorectal cancer cell line HT-29 induced by vanillin. Cancer Epidemiol 33:155–160. https://doi.org/10.1016/j.canep.2009.06.003
Hordyjewska A, Ostapiuk A, Horecka A (2018) Betulin and betulinic acid in cancer research. J Pre Clinical Clin Res 12:72–75. https://doi.org/10.26444/jpccr/92743
Huang L, Li J, Ye H et al (2012) Molecular characterization of the pentacyclic triterpenoid biosynthetic pathway in Catharanthus roseus. Planta 236:1571–1581. https://doi.org/10.1007/s00425-012-1712-0
Huguet A, del Carmen Recio M, Manez S et al (2000) Effect of triterpenoids on the inflammation induced by protein kinase C activators, neuronally acting irritants and other agents. Eur J Pharmacol 410:69–81
Hwang BY, Chai H-B, Kardono LBS et al (2003) Cytotoxic triterpenes from the twigs of Celtis philippinensis. Phytochemistry 62:197–201
Jae SP, Si HR, Dae KK et al (2009) Anti-cancer effect of betulin on a human lung cancer cell line: a pharmacoproteomic approach using 2 D SDS PAGE coupled with nano-HPLC tandem mass spectrometry. Planta Med 75:127–131. https://doi.org/10.1055/s-0028-1088366
Jäger S, Laszczyk MN, Scheffler A (2008) A preliminary pharmacokinetic study of betulin, the main pentacyclic triterpene from extract of outer Bark of Birch (Betulae alba cortex). Molecules. https://doi.org/10.3390/molecules13123224
Jäger S, Trojan H, Kopp T et al (2009) Pentacyclic triterpene distribution in various plants—rich sources for a new group of multi-potent plant extracts. Molecules 14:2016–2031
Jung G-R, Kim K-J, Choi C-H et al (2007) Effect of betulinic acid on anticancer drug-resistant colon cancer cells. Basic Clin Pharmacol Toxicol 101:277–285. https://doi.org/10.1111/j.1742-7843.2007.00115.x
Kalluri R, Weinberg RA (2009) The basics of epithelial-mesenchymal transition. J Clin Invest 119:1420–1428. https://doi.org/10.1172/JCI39104
Kasperczyk H, La Ferla-Bruhl K, Westhoff MA et al (2005) Betulinic acid as new activator of NF-kappaB: molecular mechanisms and implications for cancer therapy. Oncogene 24:6945–6956. https://doi.org/10.1038/sj.onc.1208842
Kazakova OB, Giniyatullina GV, Yamansarov EY, Tolstikov GA (2010) Betulin and ursolic acid synthetic derivatives as inhibitors of Papilloma virus. Bioorg Med Chem Lett 20:4088–4090. https://doi.org/10.1016/j.bmcl.2010.05.083
Kessler JH, Mullauer FB, de Roo GM, Medema JP (2007) Broad in vitro efficacy of plant-derived betulinic acid against cell lines derived from the most prevalent human cancer types. Cancer Lett 251:132–145. https://doi.org/10.1016/j.canlet.2006.11.003
Kim DS, Chen Z, van Nguyen T et al (1997) A concise semi-synthetic approach to betulinic acid from betulin. Synth Commun 27:1607–1612
Kim DS, Pezzuto JM, Pisha E (1998) Synthesis of betulinic acid derivatives with activity against human melanoma. Bioorg Med Chem Lett 8:1707–1712
Kim JY, Koo HM, Kim DS (2001) Development of C-20 modified betulinic acid derivatives as antitumor agents. Bioorg Med Chem Lett 11:2405–2408
Kovalenko LP, Shipaeva EV, Balakshin VV et al (2009) Antiallergenic activity of birch bark dry extract with at least 70% betulin content. Pharm Chem J 43:110–114
Krasutsky PA (2006) Birch bark research and development. Nat Prod Rep 23:919–942. https://doi.org/10.1039/b606816b
Król SK, Kielbus Michałand Rivero-Müller A, Stepulak A (2015) Comprehensive review on betulin as a potent anticancer agent. Biomed Res Int. https://doi.org/10.1155/2015/584189
Kumar V, Rani N, Aggarwal P et al (2008) Synthesis and cytotoxic activity of heterocyclic ring-substituted betulinic acid derivatives. Bioorg Med Chem Lett 18:5058–5062. https://doi.org/10.1016/j.bmcl.2008.08.003
Kutkowska J, Strzadala L, Rapak A (2017) Synergistic activity of sorafenib and betulinic acid against clonogenic activity of non-small cell lung cancer cells. Cancer Sci 108:2265–2272. https://doi.org/10.1111/cas.13386
Kuznetsova S, Skvortsova G, Malyar Y, et al (2014) Extraction of betulin from birch bark and study of its physico-chemical and pharmacological properties
Kwon HJ, Shim JS, Kim JH et al (2002) Betulinic acid inhibits growth factor-induced in vitro angiogenesis via the modulation of mitochondrial function in endothelial cells. Jpn J cancer Res GANN 93:417–425
Laavola M, Haavikko R, Hamalainen M et al (2016) Betulin derivatives effectively suppress inflammation in vitro and in vivo. J Nat Prod 79:274–280. https://doi.org/10.1021/acs.jnatprod.5b00709
Laszczyk MN (2009) Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med 75:1549–1560. https://doi.org/10.1055/s-0029-1186102
Li J, Zhang Y (2014) Increase of betulinic acid production in Saccharomyces cerevisiae by balancing fatty acids and betulinic acid forming pathways. Appl Microbiol Biotechnol 98:3081–3089. https://doi.org/10.1007/s00253-013-5461-1
Li J, Zhang Y (2015) Modulating betulinic acid production in Saccharomyces cerevisiae by managing the intracellular supplies of the co-factor NADPH and oxygen. J Biosci Bioeng 119:77–81. https://doi.org/10.1016/j.jbiosc.2014.06.013
Li Y, He K, Huang Y et al (2010) Betulin induces mitochondrial cytochrome c release associated apoptosis in human cancer cells. Mol Carcinog 49:630–640. https://doi.org/10.1002/mc.20638
Li Q, Li Y, Wang X et al (2011) Co-treatment with ginsenoside Rh2 and betulinic acid synergistically induces apoptosis in human cancer cells in association with enhanced capsase-8 activation, bax translocation, and cytochrome c release. Mol Carcinog 50:760–769. https://doi.org/10.1002/mc.20673
Liby KT, Yore MM, Sporn MB (2007) Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer 7:357–369. https://doi.org/10.1038/nrc2129
Lin WY, Lin FH, Sadhasivam S, Savitha S (2010) Antioxidant effects of betulin on porcine chondrocyte behavior in gelatin/C6S/C4S/HA modified tricopolymer scaffold. Mater Sci Eng C 30:597–604. https://doi.org/10.1016/j.msec.2010.02.010
Liu Y, Cao Y, Zhang W et al (2012) A small-molecule inhibitor of glucose transporter 1 downregulates glycolysis, induces cell-cycle arrest, and inhibits cancer cell growth in vitro and in vivo. Mol Cancer Ther 11:1672–1682. https://doi.org/10.1158/1535-7163.MCT-12-0131
Liu C-M, Qi X-L, Yang Y-F, Zhang X (2016) Betulinic acid inhibits cell proliferation and fibronectin accumulation in rat glomerular mesangial cells cultured under high glucose condition. Biomed Pharmacother 80:338–342. https://doi.org/10.1016/j.biopha.2016.02.040
Luo R, Fang D, Chu P et al (2016) Multiple molecular targets in breast cancer therapy by betulinic acid. Biomed Pharmacother 84:1321–1330. https://doi.org/10.1016/j.biopha.2016.10.018
Madonna G, Ullman CD, Gentilcore G et al (2012) NF-kappaB as potential target in the treatment of melanoma. J Transl Med 10:53. https://doi.org/10.1186/1479-5876-10-53
Malumbres M, Barbacid M (2009) Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer 9:153
Melzig MF, Bormann H (1998) Betulinic acid inhibits aminopeptidase N activity. Planta Med 64:655–657
Miller RP, Tadagavadi RK, Ramesh G, Reeves WB (2010) Mechanisms of cisplatin nephrotoxicity. Toxins (Basel) 2:2490–2518. https://doi.org/10.3390/toxins2112490
Moghaddam MG, Ahmad FBH, Samzadeh-Kermani A (2012) Biological activity of betulinic acid: a review. Pharmacol Pharm 03:119–123. https://doi.org/10.4236/pp.2012.32018
Muceniece R, Saleniece K, Rumaks J et al (2008) Betulin binds to gamma-aminobutyric acid receptors and exerts anticonvulsant action in mice. Pharmacol Biochem Behav 90:712–716. https://doi.org/10.1016/j.pbb.2008.05.015
Mullauer FB, Kessler JH, Medema JP (2009) Betulin is a potent anti-tumor agent that is enhanced by cholesterol. PLoS ONE 4:e1. https://doi.org/10.1371/journal.pone.0005361
Nazaruk J, Borzym-Kluczyk M (2015) The role of triterpenes in the management of diabetes mellitus and its complications. Phytochem Rev 14:675–690. https://doi.org/10.1007/s11101-014-9369-x
Oh S, Choi J, Lim S (2006) Protection of betulin against cadmium-induced apoptosis in hepatoma cells. Toxicology 220:1–12. https://doi.org/10.1016/j.tox.2005.08.025
Okada K (2011) The biosynthesis of isoprenoids and the mechanisms regulating it in plants. Biosci Biotechnol Biochem 75:1219–1225. https://doi.org/10.1271/bbb.110228
Orchel A, Kulczycka A, Chodurek E, et al (2014) Influence of betulin and 28-O-propynoylbetulin on proliferation and apoptosis of human melanoma. Wpływ betuliny i 28-0-propynoilobetuliny na proliferację i apoptozę ludzkich komórek czerniaka linii G-361. 191–197
Otto T, Sicinski P (2017) Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer 17:93
Ouyang L, Shi Z, Zhao S et al (2012) Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif 45:487–498. https://doi.org/10.1111/j.1365-2184.2012.00845.x
Pandey MK, Sung B, Aggarwal BB (2010) Betulinic acid suppresses STAT3 activation pathway through induction of protein tyrosine phosphatase SHP-1 in human multiple myeloma cells. Int J Cancer 127:282–292. https://doi.org/10.1002/ijc.25059
Park SY, Kim HJ, Kim KR et al (2014) Betulinic acid, a bioactive pentacyclic triterpenoid, inhibits skeletal-related events induced by breast cancer bone metastases and treatment. Toxicol Appl Pharmacol 275:152–162. https://doi.org/10.1016/j.taap.2014.01.009
Patlolla JMR, Rao CV (2012) Triterpenoids for cancer prevention and treatment: current status and future prospects. Curr Pharm Biotechnol 13:147–155. https://doi.org/10.2174/138920112798868719
Pavlova NI, Savinova OV, Nikolaeva SN et al (2003) Antiviral activity of betulin, betulinic and betulonic acids against some enveloped and non-enveloped viruses. Fitoterapia 74:489–492
Petrovic N, Schacke W, Gahagan JR et al (2007) CD13/APN regulates endothelial invasion and filopodia formation. Blood 110:142–150. https://doi.org/10.1182/blood-2006-02-002931
Pisha E, Chai H, Lee I-S et al (1995) Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat Med 1:1046–1051. https://doi.org/10.1038/nm1095-1046
Plati J, Bucur O, Khosravi-Far R (2008) Dysregulation of apoptotic signaling in cancer: molecular mechanisms and therapeutic opportunities. J Cell Biochem 104:1124–1149
Potze L, Mullauer FB, Colak S et al (2014) Betulinic acid-induced mitochondria-dependent cell death is counterbalanced by an autophagic salvage response. Cell Death Dis 5:e1169
Prakash Chaturvedula VS, Schilling JK, Johnson RK, Kingston DGI (2003) New cytotoxic lupane triterpenoids from the twigs of Coussarea paniculata. J Nat Prod 66:419–422. https://doi.org/10.1021/np0204848
Prasad S, Ravindran J, Aggarwal BB (2010) NF-kappaB and cancer: how intimate is this relationship. Mol Cell Biochem 336:25–37. https://doi.org/10.1007/s11010-009-0267-2
Rabi T, Bishayee A (2009) Terpenoids and breast cancer chemoprevention. Breast Cancer Res Treat 115:223–239. https://doi.org/10.1007/s10549-008-0118-y
Rabi T, Shukla S, Gupta S (2008) Betulinic acid suppresses constitutive and TNFalpha-induced NF-kappaB activation and induces apoptosis in human prostate carcinoma PC-3 cells. Mol Carcinog 47:964–973. https://doi.org/10.1002/mc.20447
Ragasa C, Cornelio K (2013) Triterpenes from Euphorbia hirta and their cytotoxicity. Chin J Nat Med 11:528–533
Raghuvar Gopal DV, Narkar AA, Badrinath Y et al (2005) Betulinic acid induces apoptosis in human chronic myelogenous leukemia (CML) cell line K-562 without altering the levels of Bcr–Abl. Toxicol Lett 155:343–351. https://doi.org/10.1016/j.toxlet.2004.06.015
Rastogi S, Pandey MM, Rawat AKS (2015) Medicinal plants of the genus Betula—traditional uses and a phytochemical–pharmacological review. J Ethnopharmacol 159:62–83
Retzlaff F (1902) Ueber herba gratiolae. Arch Pharm (Weinheim) 240:561–568. https://doi.org/10.1002/ardp.19022400802
Rieber M, Rieber MS (1998) Induction of p53 without increase in p21WAFl in betulinic acid-mediated cell death is preferential for human metastatic. Melanoma 17:399–406
Ruzicka L (1953) The isoprene rule and the biogenesis of terpenic compounds. Cell Mol Life Sci 9:357–367. https://doi.org/10.1007/BF02167631
Ryu SY, Lee C-K, Lee CO et al (1992) Antiviral triterpenes from Prunella vulgaris. Arch Pharm Res 15:242–245. https://doi.org/10.1007/BF02974063
Rzeski W, Stepulak A, Szymañski M et al (2006) Betulinic acid decreases expression of bcl-2 and cyclin D1, inhibits proliferation, migration and induces apoptosis in cancer cells. Naunyn Schmiedebergs Arch Pharmacol 374:11–20. https://doi.org/10.1007/s00210-006-0090-1
Rzeski W, Stepulak A, Szymański M et al (2009) Betulin elicits anti-cancer effects in tumour primary cultures and cell lines in vitro. Basic Clin Pharmacol Toxicol 105:425–432. https://doi.org/10.1111/j.1742-7843.2009.00471.x
Sarek J, Kvasnica M, Urban M et al (2005) Correlation of cytotoxic activity of betulinines and their hydroxy analogues. Bioorg Med Chem Lett 15:4196–4200. https://doi.org/10.1016/j.bmcl.2005.06.087
Sawada N, Kataoka K, Kondo K et al (2004) Betulinic acid augments the inhibitory effects of vincristine on growth and lung metastasis of B16F10 melanoma cells in mice. Br J Cancer 90:1672–1678. https://doi.org/10.1038/sj.bjc.6601746
Schmidt ML, Kuzmanoff KL, Ling-Indeck L, Pezzuto JM (1997) Betulinic acid induces apoptosis in human neuroblastoma cell lines. Eur J Cancer 33:2007–2010
Selzer E, Pimentel E, Wacheck V et al (2000) Effects of betulinic acid alone and in combination with irradiation in human melanoma cells. J Invest Dermatol 114:935–940
Shamsabadipour S, Ghanadian M, Saeedi H et al (2013) Triterpenes and steroids from euphorbia denticulata lam. With anti-herpes symplex virus activity. Iran J Pharm Res 12:759–767
Shikov AN, Djachuk GI, Sergeev DV et al (2011) Birch bark extract as therapy for chronic hepatitis C—a pilot study. Eur J Integr Med 18:807–810. https://doi.org/10.1016/j.phymed.2011.01.021
Shin YG, Cho KH, Chung SM et al (1999) Determination of betulinic acid in mouse blood, tumor and tissue homogenates by liquid chromatography-electrospray mass spectrometry. J Chromatogr B Biomed Sci Appl 732:331–336
Shin S-J, Park C-E, Baek N-I et al (2009) Betulinic and oleanolic acids isolated from Forsythia suspensa Vahl inhibit urease activity of Helicobacter pylori. Biotechnol Bioprocess Eng 14:140–145
Shin J, Lee HJ, Jung DB et al (2011) Suppression of STAT3 and HIF-1 alpha mediates anti-angiogenic activity of betulinic acid in hypoxic PC-3 prostate cancer cells. PLoS ONE 6:1–8. https://doi.org/10.1371/journal.pone.0021492
Silchenko A, Kalinovsky A, Avilov S et al (2012) Triterpene glycosides from the sea cucumber Eupentacta fraudatrix Structure and biological activity of cucumariosides B-1 and B-2, two new minor non-sulfated unprecedented triosides. Nat Prod Commun 7:1157–1162
Šiman P, Filipová A, Tichá A et al (2016) Effective method of purification of betulin from birch bark: the importance of its purity for scientific and medicinal use. PLoS ONE 11:1–14. https://doi.org/10.1371/journal.pone.0154933
So HM, Eom HJ, Lee D et al (2018) Bioactivity evaluations of betulin identified from the bark of Betula platyphylla var. japonica for cancer therapy. Arch Pharm Res 41:815–822. https://doi.org/10.1007/s12272-018-1064-9
Soica C, Dehelean C, Danciu C et al (2012) Betulin complex in γ-cyclodextrin derivatives: properties and antineoplasic activities in in vitro and in vivo tumor models. Int J Mol Sci 13:14992–15011. https://doi.org/10.3390/ijms131114992
Şoica CM, Dehelean CA, Peev C et al (2012) Physico-chemical comparison of betulinic acid, betulin and birch bark extract and in vitro investigation of their cytotoxic effects towards skin epidermoid carcinoma (A431), breast carcinoma (MCF7) and cervix adenocarcinoma (HeLa) cell lines. Nat Prod Res 26:968–974. https://doi.org/10.1080/14786419.2010.545352
Sousa MC, Varandas R, Santos RC et al (2014) Antileishmanial activity of semisynthetic lupane triterpenoids betulin and betulinic acid derivatives: synergistic effects with miltefosine. PLoS ONE 9:e89939. https://doi.org/10.1371/journal.pone.0089939
Steele JC, Warhurst DC, Kirby GC, Simmonds MS (1999) In vitro and in vivo evaluation of betulinic acid as an antimalarial. Phytother Res 13:115–119. https://doi.org/10.1002/(SICI)1099-1573(199903)13:2%3c115:AID-PTR404%3e3.0.CO;2-1
Tan Y, Yu R, Pezzuto JM (2003) Betulinic acid-induced programmed cell death in human melanoma cells involves mitogen-activated protein kinase activation. Clin Cancer Res 9:2866–2875
Tang J, Jones SA, Jeffery JL et al (2014) Synthesis and biological evaluation of macrocyclized betulin derivatives as a novel class of anti-HIV-1 maturation inhibitors. Open Med Chem J 8:23–27. https://doi.org/10.2174/1874104501408010023
Theo A, Masebe T, Suzuki Y et al (2009) Peltophorum africanum, a traditional South African medicinal plant, contains an anti HIV-1 constituent, betulinic acid. Tohoku J Exp Med 217:93–99. https://doi.org/10.1620/tjem.217.93
Thurnher D, Turhani D, Pelzmann M et al (2003) Betulinic acid: a new cytotoxic compound against malignant head and neck cancer cells. Head Neck 25:732–740. https://doi.org/10.1002/hed.10231
Tiwari R, Puthli A, Balakrishnan S et al (2014) Betulinic acid-induced cytotoxicity in human breast tumor cell lines MCF-7 and T47D and its modification by tocopherol. Cancer Invest 32:402–408. https://doi.org/10.3109/07357907.2014.933234
Tsai J-C, Peng W-H, Chiu T-H et al (2011) Anti-inflammatory effects of Scoparia dulcis L. and betulinic acid. Am J Chin Med 39:943–956. https://doi.org/10.1142/S0192415X11009329
Urban M, Vlk M, Dzubak P et al (2012) Cytotoxic heterocyclic triterpenoids derived from betulin and betulinic acid. Bioorg Med Chem 20:3666–3674. https://doi.org/10.1016/j.bmc.2012.03.066
Viji V, Helen A, Luxmi VR (2011) Betulinic acid inhibits endotoxin-stimulated phosphorylation cascade and pro-inflammatory prostaglandin E2 production in human peripheral blood mononuclear cells. Br J Pharmacol 162:1291–1303. https://doi.org/10.1111/j.1476-5381.2010.01112.x
Wachsberger PR, Burd R, Wahl ML, Leeper DB (2002) Betulinic acid sensitization of low pH adapted human melanoma cells to hyperthermia. Int J Hyperth 18:153–164
Wada S, Tanaka R (2005) Betulinic acid and its derivatives, potent DNA topoisomerase II inhibitors, from the bark of Bischofia javanica. Chem Biodivers 2:689–694. https://doi.org/10.1002/cbdv.200590045
Wan Y, Wu Y-L, Lian L-H et al (2012) The anti-fibrotic effect of betulinic acid is mediated through the inhibition of NF-kappaB nuclear protein translocation. Chem Biol Interact 195:215–223. https://doi.org/10.1016/j.cbi.2012.01.002
Wang D-Y, Liu J, Yin M-Z et al (2012) Betulin induces apoptosis of HeLa cell lines in vitro and its possible mechanism. Tumor 32:234–238
Weigelt B, Peterse JL, van’t Veer L (2005) Breast cancer metastasis: markers and models. Nat Rev Cancer 5:591–602. https://doi.org/10.1038/nrc1670
Woldemichael GM, Singh MP, Maiese WM, Timmermann BN (2003) Constituents of antibacterial extract of Caesalpinia paraguariensis Burk. Z Naturforsch C 58:70–75
Wu J, Niu Y, Bakur A et al (2017) Cell-free production of pentacyclic triterpenoid compound betulinic acid from betulin by the engineered Saccharomyces cerevisiae. Molecules. https://doi.org/10.3390/molecules22071075
Yang L, Chen Y, Ma Q et al (2010) Effect of betulinic acid on the regulation of Hiwi and cyclin B1 in human gastric adenocarcinoma AGS cells. Acta Pharmacol Sin 31:66–72. https://doi.org/10.1038/aps.2009.177
Yogeeswari P, Sriram D (2005) Betulinic acid and its derivatives: a review on their biological properties. Curr Med Chem 12:657–666. https://doi.org/10.2174/0929867053202214
Zdzisinska B, Rzeski W, Paduch R et al (2003) Differential effect of betulin and betulinic acid on cytokine production in human whole blood cell cultures. Pol J Pharmacol 55:235–238
Zdzisińska B, Szuster-Ciesielska A, Rzeski W, Kanderfer-Szerszen M (2010) Therapeutic properties of betulin and betulinic acid, components of birch bark extract. Farmaceutyczny Przeglad Naukowy 7(3):33–39
Zeng A-Q, Yu Y, Yao Y-Q et al (2018) Betulinic acid impairs metastasis and reduces immunosuppressive cells in breast cancer models. Oncotarget 9:3794–3804. https://doi.org/10.18632/oncotarget.23376
Zhang D, Xu H, Wang L et al (2015) The influenza virus polymerase complex: an update on its structure, functions, and significance for antiviral drug design. Med Res Rev 35:1127–1155. https://doi.org/10.1002/med
Zhang X, Hu J, Chen Y (2016) Betulinic acid and the pharmacological effects of tumor suppression (review). Mol Med Rep 14:4489–4495. https://doi.org/10.3892/mmr.2016.5792
Zhao Z, Wang J, Tang J et al (2012) JNK- and Akt-mediated Puma expression in the apoptosis of cisplatin-resistant ovarian cancer cells. Biochem J 444:291–301. https://doi.org/10.1042/BJ20111855
Zhou C, Li J, Li C, Zhang Y (2016) Improvement of betulinic acid biosynthesis in yeast employing multiple strategies. BMC Biotechnol 16:1–9. https://doi.org/10.1186/s12896-016-0290-9
Zhou Z, Zhu C, Cai Z, Zhao F (2018) Betulin induces cytochrome c release and apoptosis in colon cancer cells via NOXA. Oncol Lett. https://doi.org/10.3892/ol.2018.8183
Zhuo Z-J, Xiao M-J, Lin H-R et al (2018) Novel betulin derivative induces anti-proliferative activity by G2/M phase cell cycle arrest and apoptosis in Huh7 cells. Oncol Lett 15:2097–2104. https://doi.org/10.3892/ol.2017.7575
Zuco V, Supino R, Righetti SC et al (2002) Selective cytotoxicity of betulinic acid on tumor cell lines, but not on normal cells. Cancer Lett 175:17–25. https://doi.org/10.1016/S0304-3835(01)00718-2
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hordyjewska, A., Ostapiuk, A., Horecka, A. et al. Betulin and betulinic acid: triterpenoids derivatives with a powerful biological potential. Phytochem Rev 18, 929–951 (2019). https://doi.org/10.1007/s11101-019-09623-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-019-09623-1