Abstract
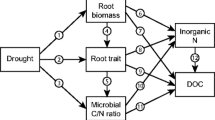
This study examined how root growth and morphology were affected by variation in soil moisture at four Amazon rainforest sites with contrasting vegetation and soil types. Mean annual site root mass, length and surface area growth ranged between 3–7 t ha−1, 2–4 km m−2 and 8–12 m2 m−2 respectively. Mean site specific root length and surface area varied between 8–10 km kg−1 and 24–34 m2 kg−1. Growth of root mass, length and surface area was lower when soil water was depleted (P < 0.001) while specific root length and surface area showed the opposite pattern (P < 0.001). These results indicate that changes in root length and surface area per unit mass, and pulses in root growth to exploit transient periods of high soil water availability may be important means for trees in this ecosystem to increase nutrient and water uptake under seasonal and longer-term drought conditions.





Similar content being viewed by others
References
Andreae MO, Rosenfeld D, Artaxo P, Costa AA, Frank GP, Longo KM, Silva-Dias MAF (2004) Smoking rain clouds over the Amazon. Science 303:1337–1342
Atkin OK, Edwards EJ, Loveys BR (2000) Response of root respiration to changes in temperature and its relevance to global warming. New Phytol 147:141–154
Berish CW (1982) Root biomass and surface area in three successional tropical forests. Can J Res 12:699–704
Bengough AG, Bransby MF, Hans J, McKenna SJ, Roberts TJ, Valentine TA (2006) Root responses to soil physical conditions; growth dynamics from field to cell. J Exp Bot 57:437–447
Bingham IJ, Bengough AG (2003) Morphological plasticity of wheat and barley roots in response to spatial variation in soil strength. Plant Soil 250:273–282
Cannell MGR, Dewar RC (1994) Carbon allocation in trees: a review of concepts for modelling. Adv Ecol Res 25:59–104
Carvalheiro K, Nepstad D (1996) Deep soil heterogeneity and fine root distribution in forests and pastures of eastern Amazônia. Plant Soil 182:279–285
Cavelier J, Wright SJ, Santamaria J (1999) Effects of irrigation, fine root biomass and production in a semideciduous lowland forest in Panama. Plant and Soil 211:207–213
Comas LH, Anderson LJ, Dunst RM, Lakso AN, Eissenstat DM (2005) Canopy and environmental control of root dynamics in a long-term study of Concord grape. New Phytol 167:829–840
Costa MH, Foley JA (2000) Combined effects of deforestation and doubled atmospheric CO2 concentrations on the climate of Amazonia. J Clim 13:18–34
Cuevas E, Medina E (1988) Nutrient dynamics in Amazonian forests. II. Fine root growth, nutrient availability and leaf litter decomposition. Oecologia 76:222–235
Davis JP, Haines B, Coleman D, Hendrick R (2004) Fine root dynamics along an elevational gradient in the southern Appalachian Mountains, USA. For Ecol Manage 187:19–34
Dickman DI, Nguyen PV, Pregitzer KS (1996) Effects of irrigation and coppicing on above-ground growth, physiology, and fine-root dynamics of two field-grown hybrid poplar clones. For Ecol Manage 80:163–174
Eissenstat DM, Wells CE, Yanai RD, Whitbeck JL (2000) Building roots in a changing environment: implications for root longevity. New Phytol 147:33–42
Farquhar GD, Von Caemmerer S (1982) Modelling of photosynthetic response to the environment Physiological Plant Ecology II. In: Lange OL, Nobel PS, Osmond CB, Ziegler H (eds) Encyclopedia of plant physiology. New Series, Springer, Berlin, pp 549–587
Farrish KW (1991) Spatial and temporal fine-root distribution in three Louisiana forest soils. Soil Sci Soc Am J 55:1752–1757
Fernandez OA, Caldwell MM (1975) Phenology and dynamics of root growth of three cool semi-desert shrubs under field conditions. J Ecol 63:703–714
Fisher RA, Williams M, do Vale RL, da Costa AL, Meir P (2006) Evidence from Amazonian forests is consistent with isohydric control of leaf water potential. Plant Cell Environ 29:151–165
Fisher RA, Williams M, Lola da Costa A, Malhi M, da Costa RF, Almeida S, Meir PW (2007). The response of an eastern Amazonian rain forest to drought stress: results and modelling analyses from a through-fall exclusion experiment. Glob Change Biol 13:2361–2378
Fitter AH, Heinemeyer A, Staddon PL (2000) The impact of elevated CO2 and global climate change on arbuscular mycorrhizas: a mycocentric approach. New Phytol 147:179–187
Hendrick RL, Pregitzer KS (1993) Patterns of fine root mortality in two sugar maple forests. Nature 361:59–61
Hendrick RL, Pregitzer KS (1996) Temporal and depth-related patterns of fine root dynamics in northern hardwood forests. J Ecol 84:167–176
Hendrick RL, Pregitzer KS (1997) The relationship between fine root demography and soil environment in northern hardwood forests. Ecoscience 4:99–105
Hendricks JJ, Hendrick RL, Wilson CA, Mitchell RJ, Pecot SD, Guo D (2006) Assessing the patterns and controls of fine root dynamics: an empirical test and methodological review. J Ecol 94:40–57
Houghton RA, Lawrence KT, Hackler JL, Brown S (2001) The spatial distribution of forest biomass in the Brazilian Amazon: a comparison of estimates. Glob Change Biol 7:731–746
Intergovernmental Panel for Climate Change (2001) Climate Change 2001: the scientific basis. In: Contribution of working group I to the third assessment report of the International Panel on Climate Change, Cambridge University Press
Itoh S (1985) In situ measurement of rooting density by micro-rhizotron. Soil Sci Plant Nutr 31:653–656
Jackson RB, Mooney HA, Schulze ED (1997) A global budget for fine root biomass, surface area, and nutrient contents. Proc Natl Acad Sci U S A 94:7362–7366
Jordan CF, Escalante G (1980) Root productivity in an Amazonian rain forest. Ecology 61:14–18
Joslin JD, Wolfe MH, Hanson PJ (2000) Effects of altered water regimes on forest root systems. New Phytol 147:117–129
Lehmann J, Kern DC, Glaser B, Woods WI (2003) Amazonian Dark Earths: Origin, Properties, Management. Kluwer Academic Publishers, Dordrecht, Netherlands
Meisner CA, Karnok KJ (1992) Peanut root response to drought stress. Agron J 84:159–165
Metcalfe DB, Meir P, Aragão LEOC, Malhi M, da Costa ACL, Braga A, Gonçalves PHL, de Athaydes J, de Almeida SS, Williams M (2007a) Factors controlling spatio-temporal variation in carbon dioxide efflux from surface litter, roots and soil organic matter at four rain forest sites in the eastern Amazon. Journal of Geophysical Research-Biogeosciences 112, G04001, doi:10.1029/2007JG000443
Metcalfe DB, Meir P, Williams M (2007b) A comparison of methods for converting rhizotron root length measurements into estimates of root mass production per unit ground area. Plant Soil 301:279–288
Metcalfe DB, Williams M, Aragão LEOC, Da Costa ACL, De Almeida SS, Braga AP, Gonçalves PHL, De Athaydes Silva Junior, Malhi Y, Meir P (2007c) A method for extracting plant roots from soil which facilitates rapid samples processing without compromising measurement accuracy. New Phytol 174:697–703
Moutinho P, Nepstad D, Davidson DE (2003) Cutter ant Atta sexdens effects on soil, root distribution, and tree growth in a secondary forest of eastern Amazonia. Ecology 84:1265–1276
Nadelhoffer KJ, Raich JW (1992) Fine root production estimates and belowground carbon allocation in forest ecosystems. Ecology 73:1139–1147
Norby RJ, Jackson RB (2000) Root dynamics and global change: seeking an ecosystem perspective. New Phytol 147:3–12
Pregitzer KS, King JS, Burton AJ, Brown SS (2000) Responses of tree fine roots to temperature. New Phytol 147:105–115
Priess J, Then C, Folster H (1999) Litter and fine root production in three types of tropical premontane rain forest in SE Venezuela. Plant Ecol 143:171–187
Roderstein M, Hertel D, Leuschner C (2005) Above- and below-ground litter production in three tropical montane forests in southern Ecuador. J Trop Ecol 21:483–492
Ruivo MLP, Cunha ES (2003) Mineral and organic components in archaeological black earth and yellow latosol in Caxiuanã, Amazon, Brazil. In: Tiezzi E, Brebbia CA, Uso JL (eds) Ecosystems and sustainable development. WIT, Southampton, pp 1113–1121
Sanford RL (1990) Fine root biomass under light gap openings in an Amazon rain forest. Oecologia 83:541–545
Schoengart J, Junk WJ, Piedade MTF, Ayres JM, Hutterman A, Worbes M (2004) Teleconnection between tree growth in the Amazonian floodplains and the Niño-Southern Oscillation effect. Glob Change Biol 10:683–692
Shukla J, Nobre C, Sellers P (1990) Amazon deforestation and climate change. Science 247:1322–1325
Schwarz PA, Law BE, Williams M, Irvine J, Kurpius M, Moore D (2004) Climatic versus biotic constraints on carbon and water fluxes in seasonally drought-affected ponderosa pine ecosystems. Glob Biogeochem Cycles doi:101029/2004GB002234
Sierra CA, Harmon ME, Moreno FH, Orrego SA, del Valle JI (2007) Spatial and temporal variability of net ecosystem production in a tropical forest: testing the hypothesis of a significant carbon sink. Glob Change Biol 13:838.853
Silva Dias MAF, Rutledge S, Kabat P, Dias PLS, Nobre C, Fisch G et al (2002) Cloud and rain processes in a biosphere-atmosphere interaction context in the Amazon region. J Geophys Res-Atmos 107:8072–8084
Silver WL, Thompson AW, Mcgroddy ME, Varner RK, Dias JD, Silva H et al (2005) Fine root dynamics and trace gas fluxes in two lowland tropical forest soils. Glob Change Biol 11:290–306
Sotta ED (2006) Soil carbon dioxide dynamics and nitrogen cycling in an eastern Amazonian rainforest, Caxiuana, Brazil. Ph.D. thesis, Georg-August-University of Göttingen, Germany
Steingrobe B, Schmid H, Claassen N (2000) The use of the ingrowth core method for measuring root production of arable crops—influence of soil conditions inside the ingrowth core on root growth. J Plant Nutr Soil Sci 163:617–622
Sword MA, Gravatt DA, Faulkner PL, Chambers JL (1996) Seasonal branch and fine root growth of juvenile Loblolly pine five growing seasons after fertilization. Tree Physiol 16:899–904
Thornley JHM (1972) A balanced quantitative model for root:shoot ratios in vegetative plants. Ann Bot (Lond) 36:431–441
Torreano SJ, Morris LA (1998) Loblolly pine root growth and distribution under water stress. Soil Sci Soc Am J 62:818–827
Trenberth KE, Hoar TJ (1997) El Niño and climate change. Geophys Res Lett 24:3057–3060
Trumbore SE, Gaudinski JB (2003) The secret lives of roots. Science 302:1344–1345
Vogt KA, Vogt DJ, Bloomfield J (1998) Analysis of some direct and indirect methods of estimating root biomass and production of forests at an ecosystem level. Plant Soil 200:71–89
West JB, Espeleta JF, Donovan LA (2003) Root longevity and phenology differences between two co-occurring savanna bunchgrasses with different leaf habits. Funct Ecol 17:20–28
Whalley WR, Bengough AG, Dexter AR (1998) Water stress induced by PEG decreases the maximum growth pressure of the roots of pea seedlings. J Exp Bot 49:1689–1694
Williams M, Malhi Y, Nobre AD, Rastetter EB, Grace J, Pereira MGP (1998) Seasonal variation in net carbon exchange and evapotranspiration in a Brazilian rain forest: a modelling analysis. Plant Cell Environ 21:953–968
Zak DR, Pregitzer KS, King JS, Holmes WE (2000) Elevated atmospheric CO2, fine roots and the response of soil micro-organisms: a review and hypothesis. New Phytol 147:201–222
Acknowledgements
This research contributes to the Brazil-led Large Scale Biosphere—Atmosphere Experiment in Amazonia. Work was supported by a Natural Environment Research Council (UK) PhD studentship and a Standard Research Grant (NER/A/S/2003/1609), a Royal Society (UK) Dudley Stamp Memorial Fund award, and an Elizabeth Sinclair Fund award (School of Geosciences, University of Edinburgh, UK). The authors would like to thank Leonardo Sá and Ima Vieira for their scientific support and collaboration, the Museu Paraense Emilio Goeldi for the use of its field station and laboratory facilities, and Bene and Joca for committed field work assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Yan Li.
Rights and permissions
About this article
Cite this article
Metcalfe, D.B., Meir, P., Aragão, L.E.O.C. et al. The effects of water availability on root growth and morphology in an Amazon rainforest. Plant Soil 311, 189–199 (2008). https://doi.org/10.1007/s11104-008-9670-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-008-9670-9