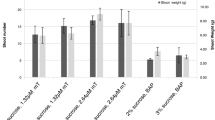
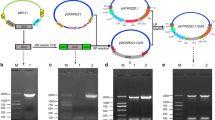
Abstract
Grafting is a common method for clonal propagation of fruit trees. Moreover, it serves as a mean to deal with abiotic stress, adjust tree growth vigor, increase yield, and improve other fruit quality traits. Investigations of rootstock and scion graft relationships have originally focused on anatomical and cellular development, nutrient transport, and hormonal movement across graft union. Discovery of long distance transport of mRNA and small RNAs in phloem tissues of rootstock and scion has provided new opportunities for investigation. In this study, we report on the endogenous transport of Gibberellic acid insensitive (GAI) across graft union of a traditional local Chinese pear cultivar, Pyrus bretschneideri cv. Yali (scion), and a wild Pyrus betulaefolia cv. Bunge (rootstock). Cleaved amplified polymorphic sequence analysis RT-PCR indicated that Pyrus-GAI can be transported within 4 and 10 days after micro-grafting, and it can also be transported to a 10–50-cm tall scion of a 2-year-old grafting tree. To further investigate the transport capacity of Pyrus-GAI transcript, a 35S:pear (P. betulaefolia)-GAI transgenic tobacco (Nicotiana tabacum L. cv. Samson.) was prepared and grafted to wild-type tobacco. RT-PCR indicated that sustained transmission of GAI mRNA through the graft union occurred from the 15th day after grafting. The results have laid a foundation for improving rootstock and regulating the properties of scion in fruit trees by transgenic technology.






Similar content being viewed by others
References
Banerjee AK, Chatterjee M, Yu Y, Suh SG, Wa M, Hannapel DJ (2006) Dynamics of a mobile RNA of potato involved in a long distance signaling pathway. Plant Cell 18:3443–3457
Banerjee AK, Lin T, Hannapel DJ (2009) Untranslated regions of a mobile transcript mediate RNA metabolism. Plant Physiol 151:1831–1843
Chang S, Puryear J, Cairney J (1993) A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep 11:113–116
Clark SE (2001) Cell signalling at the shoot meristem. Nature Reviews Mol Cell Biol 2:276–284
Deeken R, Ache P, Kajahn I, Klinkerberg J, Bringmann G, Hedrich R (2008) Identification of Arabidopsis thaliana phloem RNAs provides a search criterion for phloem-based transcripts hidden in complex datasets of microarray experiments. Plant J 55:746–759
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Gómez G, Torres H, Pallás V (2005) Identification of translocatable RNA-binding phloem proteins from melon, potential components of the long-distance RNA transport system. Plant J 41(1):107–16
Goodenough DA, Goliger JA, Paul DL (1996) Connexins, connexons, and intercellular communication. Annu Rev Biochem 65:475–502
Gurdon JB, Dyson S, Johnson D (1998) Cells’ perception of position in a concentration gradient. Cell 95:159–162
Ham B-K, Jeri L, Brandom, Xoconostle-Cázares B, Ringgold V, Lough TJ (2009) A polypyrimidine tract binding protein, pumpkin RBP50, forms the basis of a phloem-mobile ribonucleoprotein complex. Plant Cell 21:197–215
Haywood V, Yu TS, Huang NC, Lucas WJ (2005) Phloem long distance trafficking of Gibberellic Acid-Insensitive RNA regulates leaf development. Plant J 42:49–68
Huang NC, Yu TS (2009) The sequence of Arabidopsis GA insensitive RNA constitute the motifs that are necessary and sufficient for RNA long-distance trafficking. Plant J 59:921–929
Hynes LW, Peng JR, Richards DE, Harberd NP (2003) Transgenic expression of the Arabidopsis DELLA proteins GAI and gai confers altered gibberellin response in tobacco. Transgenic Res 12:707–714
Jensen PJ, Rytter J, Detwiler EA, Travis JW, Mcnellis TW (2003) Rootstock effects on gene expression patterns in apple tree scions. Plant Mol Biol 493:493–511
Jensen PJ, Makalowska I, Altman N, Fazio G, Praul C, Naximova SN, Crassweller RM, Travis JW, Mcnellis TW (2010) Rootstock regulated gene expression patterns in apple tree scion. Tree Genet Genomes 6:57–72
Kamboj JS, Blake PS, Quinlan JD, Baker DA (1999a) Identification and quantitation of GC-MS of zeatin and zeatin riboside in xylem sap from rootstock and scion of grafted apple trees. Plant Growth Regul 28:199–205
Kamboj JS, Browning G, Blake PS, Quinlan JD, Baker DA (1999b) GC-MS-SIM analysis of abscisic acid and indole-3-acetic acid in shoot bark of apple rootstocks. Plant Growth Regul 28:21–27
Kanehira A, Yamada K, Iwaya T, Tsuwamoto R, Kasai A, Nakazono M, Harada T (2009) Apple phloem cells contain some mRNAs transported over long distances. Tree Genet Genomes 6:635–642
Khan MA, Zhao Y-F, Korban SS (2011) Molecular mechanisms of pathogenesis and resistance to the bacterial pathogen Erwinia amylovora, causal agent of fire blight disease in rosaceae. Plant Mol Biol Rep. doi:10.1007/s11105-011-0334-1
Kim M, Canio W, Kessler S, Shinha N (2001) Developmental changes due to long-distance movement of a homeobox fusion transcript in tomato. Science 293:287–289
Kragler F, Yoo BC, Lucas WJ (2001) RNA as a long-distance information macromolecule in plants. Mol Cell Biol 2:849–856
Kudo H, Harada T (2007) A graft-transmissible RNA from tomato rootstock changes leaf morphology of potato scion. HortSci 42:225–226
Li C-Y, Zhang K, Zeng X-W, Jackson S, Zhou Y, and Hong Y-G (2009) A cis element within flowering locus T mRNA determines its mobility and facilitates trafficking of heterologous viral RNA. J Virology (83):3540–3548
Li F, Lei H-J, Zhao X-J, Tian R-R, Li T-H (2011) Characterization of three sorbitol transporter genes in micropropagated apple plants grown under drought stress. Plant Mol Biol Rep. doi:10.1007/s11105-011-0323-4
Lucas WJ (1995) Plasmodesmata: intercellular channels for macromolecular transport in plants. Curr Opin Cell Biol 7:673–680
Lucas WJ, Ding B, Van der Schoot C (1993) Plasmodesmata and the supracellular nature of plants. New Phytol 125:435–476
Lucas WJ, Bouché-Pillon S, Jackson DP, Nguyen L, Baker L, Ding B, Hake S (1995) Selective traffic king of KNOTTED1 homeodomain protein and its mRNA through plasmodesmata. Science 270:1980–1983
Ma J, He Y-H, Wu C-H, Liu H-P, Hu Z-Y, Sun G-M (2011) Cloning and molecular characterization of a SERK gene transcriptionally induced during somatic embryogenesis in Ananas comosus cv. Shenwan Plant Mol Biol Rep. doi:10.1007/s11105-011-0330-5
Mallory C, Ely L, Smith TH, Marathe R, Anandalakshmi R, Fagard M, Vaucheret H, Pruss G, Bowman L, Vance VB (2001) HC-Pro suppression of transgene silencing eliminates the small RNAs but not transgene methylation or the mobile signal. Plant Cell 13:571–583
Mallory C, Mlotshwa S, Bowman LH, Vance VB (2003) The capacity of transgenic tobacco to send a systemic RNA silencing signal depends on the nature of the inducing transgene locus. Plant J 35:82–92
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Rivera-Vega L, Mamidala P, Koch JL, Mason ME, Mittapalli O (2011) Evaluation of reference genes for expression studies in ash (Fraxinus spp.) Plant Mol Biol Rep doi. 10.1007/s11105-011-0340-3
Ruiz-Medrano R, Xoconostle-Cázares B, Lucas WJ (1999) Phloem and long-distance transport of CmNACP mRNA: implications for supracellular regulation in plants. Development 126:4405–4419
Samanci H, Uslu I (1995) Rootstock adaptation and affinity studies on Razaki and Muskule grape varieties. ACHRI p 30
Schmitt B, Stadler R, Sauer N (2008) Immunolocalization of solanaceous SUT1 proteins in companion cells and xylem parenchyma: new perspectives for phloem loading and transport. Plant Physiol 148:187–199
Smaka A, Li X-Y, Heikelt C, Welander M, Zhu L-H (2010) Effects of transgenic rootstocks on growth and development of non-transgenic scion cultivars in apple. Transgenic Res 19(6):933–948
Soria-Guerra RE, Rosales-Mendoza S, Gasic K, Wisniewski ME, Band M, Korban SS (2011) Gene expression is highly regulated in early developing fruit of apple. Plant Mol Biol Rep. doi:10.1007/s11105-011-0300-y
Soumelidou K, Morris DA, Battey NH, Barnett JR, John P (1994) Auxin transport capacity in relation to the dwarfing effect of apple root-stocks. J Hort Sci 69:719–725
Sui J-M, Guo B-T, Wang J-S, Qiao L-X, Zhou Y, Zhang H-G, Gu M-H, Liang G-H (2011) A new GA-insensitive semidwarf mutant of rice (Oryza sativa L.) with a missense mutation in the SDG Gene. Plant Mol Biol Rep doi. 10.1007/s11105-011-0321-6
Tränkner C, Lehmann S, Hoenicka H, Hanke M-V, Fladung M, Lenhardt D, Dunemann F, Gau A, Schlangen K, Malnoy M, Flachowsky H (2010) Overexpression of an FT-homologous gene of apple induces early flowering in annual and perennial plants. Planta 232:1309–1324
Viswanathan A, Kuriakose B, Bharadwaj S, Thomas G (2011) Expression of Aprotinin in anther causes male sterility in tobacco var Petit havana. Plant Mol Biol Rep. doi:10.1007/s11105-011-0288-3
Westwood MN (1993) Hormones and growth regulators. In: Westwood MN (ed) Temperate-zone pomology, physiology and culture, 3rd ed. Timber Press Inc, Portland, pp 364–381
Xoconostle-Cazares B, Xiang Y, Ruiz-Medrano R, Wang HL, Monzer J, Yoo BC, McFarland KC, Franceschi VR, Lucas WJ (1999) Plant paralog to viral movement protein that potentiates transport of mRNA into the phloem. Science 283:94–98
Xu H-Y, Zhang W-N, Li M-F, Harada T, Han Z-H, Li T-Z (2010) Gibberellic acid insensitive mRNA transport in both directions between stock and scion in Malus. Tree Genet Genomes 6:1013–1019
Zambounis AG, Kalamaki MS, Tani EE, Paplomatas EJ, Tsaftaris AS (2011) Expression analysis of defense-related genes in cotton (Gossypium hirsutum) after Fusarium oxysporum f. sp. vasinfectum infection and following chemical elicitation using a salicylic acid analog and methyl jasmonate. Plant Mol Biol Rep doi. 10.1007/s11105-011-0335-0
Acknowledgments
We would like to thank Song-ling Bai (Hirosaki University, Japan) and Ai-de Wang (Cornell University, USA) for the technical assistance. This work was supported by the Doctoral Program Special Fund of the Ministry of Education in China (2010000811036), National Natural Science Foundation of China (30871697) and Beijing Natural Science Foundation (6102017).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Nucleotide sequences of DL-GAI and YL-GAI genes. YL-GAI, P. bretschneideri GAI; DL-GAI, P. betulaefolia GAI (JPEG 946 kb)
Fig. S2
CAPS analysis of DL-GAI and YL-GAI alleles. a P. bretschneideri ‘Yali’ and Bunge cDNA sequences. b Predicted fragment sizes by HapII digestion (JPEG 30 kb)
Fig. S3
Evidence of no contamination of gDNA in cDNA preparations. a The genomic structure of a part of the Actin gene. Boxes and lines indicate exons and introns, respectively. The positions of primers used are shown above the structure. b PCR products when gDNA and cDNA were both used as templates. The gDNA fraction amplified the PCR product including introns, whereas the cDNA fractions amplified the 82 bp exon sequences. The scion and stock cDNA fractions were from plants sampled 5 days after grafting. M. DNA ladder 2000 (JPEG 23 kb)
Fig. S4
Transgenic construct of 35S:DL-GAI cDNA into pBI 121. The plant expression vector pBI 121 (GenBank ID, AF485783) contained 35S CaMV promoter, resistant tab neomycin phosphotransferaseII (NPTII), and a nopaline synthase (nos) 3’ transcriptional terminator sequence, was used for the expression studies in transgenic tobaccos. The sense primer XBAGAIF was contained in XbaIsite at the 5’ end and the antisense primer SACGAIR was contained in SacIsite at the 3’ end. Amplified DL-GAI cDNA sequences were purified and inserted into the same sites of pBI 121 without GUS. The pBI 121 without GUS gene was used as controls (JPEG 25 kb)
Rights and permissions
About this article
Cite this article
Zhang, WN., Gong, L., Ma, C. et al. Gibberellic Acid-Insensitive mRNA Transport in Pyrus. Plant Mol Biol Rep 30, 614–623 (2012). https://doi.org/10.1007/s11105-011-0365-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-011-0365-7