Abstract
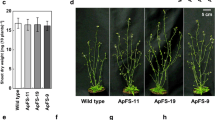
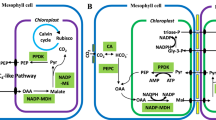
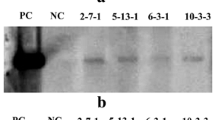
Plants with C4 photosynthesis are efficient in carbon assimilation and have an advantage over C3 photosynthesis. In C4 photosynthesis, the primary CO2 fixation is catalyzed by phosphoenolpyruvate carboxylase (PEPC). Here, we show that overexpression of Zea mays PEPC cDNA, under the control of 35S promoter, in Arabidopsis thaliana resulted in ~7–10 fold higher protein abundance and ~7–10 fold increase in PEPC activity in the transgenic lines than that in the vector control. We suggest that overexpression of PEPC played an anaplerotic role to increase the supply of 4-carbon carboxylic acids, which provided carbon skeletons for increased amino acid and protein synthesis. Higher protein content must have been responsible for increased metabolic processes including chlorophyll biosynthesis, photosynthesis, and respiration. Consequently, the PEPC-overexpressed transgenic plants had higher chlorophyll content, enhanced electron transport rate (ETR), lower non-photochemical quenching (NPQ) of chlorophyll a fluorescence, and a higher performance index (PI) than the vector control. Consistent with these observations, the rate of CO2 assimilation, the starch content, and the dry weight of PEPC-overexpressed plants increased by 14–18 %, 10–18 %, and 6.5–16 %, respectively. Significantly, transgenics were tolerant to salt stress as they had increased ability to synthesize amino acids, including the osmolyte proline. NaCl (150 mM)-treated transgenic plants had higher variable to maximum Chl a fluorescence (F v/F m) ratio, higher PI, higher ETR, and lower NPQ than the salt-treated vector controls. These results suggest that expression of C4 photosynthesis enzyme(s) in a C3 plant can improve its photosynthetic capacity with enhanced tolerance to salinity stress.









Similar content being viewed by others
Abbreviations
- APT:
-
Adenine phosphoribosyl transferase
- CaMV:
-
Cauliflower mosaic virus
- Chl:
-
Chlorophyll
- EDTA:
-
Ethylenediaminetetraacetic acid
- ETR:
-
Electron transport rate, see text for details
- F m :
-
Maximum Chl fluorescence
- F o :
-
Minimum Chl fluorescence
- F v :
-
Variable fluorescence, F m − F o
- gs:
-
Stomatal conductance
- LED:
-
Light-emitting diode
- MDA:
-
Malondialdehyde, CH2(CHO)2
- MDH:
-
Malate dehydrogenase
- ME:
-
Malic enzyme
- MS:
-
Murashige and Skoog medium
- NPQ:
-
Non-photochemical quenching (of Chl fluorescence)
- nptII:
-
Neomycin phosphotransferase (kanamycin resistance gene)
- OAA:
-
Oxaloacetic acid
- PAR:
-
Photosynthetically active radiation
- PEP:
-
Phosphoenolpyruvate
- PEPC:
-
Phosphoenolpyruvate carboxylase
- PFD:
-
Photon flux density
- PI:
-
Performance index, see text for details
- PMSF:
-
Phenylmethylsulfonyl fluoride
- PSI:
-
Photosystem I
- PSII:
-
Photosystem II
- PVP:
-
Polyvinylpyrrolidone
- qE:
-
Energy-dependent quenching of the excited state of Chl a
- qI:
-
Photoinhibitory quenching of the excited state of Chl a
- qT:
-
State transition quenching of the excited state of Chl a
- RC/ABS:
-
Density of reaction centers per PSII antenna chlorophyll (see text for details)
- RC:
-
Reaction center
- ROS:
-
Reactive oxygen species
- RuBisCO:
-
Ribulose-1,5-bisphosphate carboxylase/oxygenase
- TAE:
-
Tris-base, acetic acid and EDTA
- TEM:
-
Transmission electron microscopy
- VC:
-
Vector control
- WT:
-
Wild type
- WUE:
-
Water use efficiency
References
Agarie S, Miura A, Sumikura R, Tsukamoto S, Nose A, Arima S, Matsuoka M, Miyao-Tokutomi M (2002) Overexpression of C4 PEPC caused O2-insensitive photosynthesis in transgenic rice plants. Plant Sci 162(2):257–265
Akram NA, Ashraf M (2011) Improvement in growth, chlorophyll pigments and photosynthetic performance in salt-stressed plants of sunflower (Helianthus animus L.) by foliar application of 5-aminolevulinic acid. Agrochimica 55(2):94–104
Allen JF, Mullineaux CW (2004) Probing the mechanism of state transitions in oxygenic photosynthesis by chlorophyll fluorescence spectroscopy, kinetics and imaging. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence: a signature of photosynthesis, advances in photosynthesis and respiration, vol 19. Springer, Dordrecht, pp 447–461
Avasthi UK, Izui K, Raghavendra AS (2011) Interplay of light and temperature during the in planta modulation of C4 phosphoenolpyruvate carboxylase from the leaves of Amaranthus hypochondriacus L.: diurnal and seasonal effects manifested at molecular levels. J Exp Bot 62(3):1017–1026
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113. doi:10.1146/annurev.arplant.59.032607.092759
Bandyopadhyay A, Datta K, Zhang J, Yang W, Raychaudhuri S, Miyao M, Datta SK (2007) Enhanced photosynthesis rate in genetically engineered indica rice expressing pepc gene cloned from maize. Plant Sci 172(6):1204–1209
Basu PS, Sharma A, Sukumaran NP (1998) Changes in net photosynthetic rate and chlorophyll fluorescence in potato leaves induced by water stress. Photosynthetica 35:13–19
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39(1):205–207
Beaujean A, Issakidis-Bourguet E, Catterou M, Dubois F, Sangwan RS, Sangwan-Norreel BS (2001) Integration and expression of sorghum C4 phosphoenolpyruvate carboxylase and chloroplastic NADP+-malate dehydrogenase separately or together in C3 potato plants. Plant Sci 160:1199–1210
Biswal AK, Pattanayak GK, Pandey SS, Leelavathi S, Reddy VS, Govindjee, Tripathy BC (2012) Light intensity-dependent modulation of chlorophyll b biosynthesis and photosynthesis by overexpression of chlorophyllide a oxygenase in tobacco. Plant Physiol 159(1):433–449
Björkman O, Demmig B (1987) Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 170(4):489–504
Blankenship RE (2014) Molecular mechanisms of photosynthesis, 2nd edn. Wiley/Blackwell, Chichester
Boggess SF, Aspinall D, Paleg LG (1976) Stress metabolism. IX. The significance of end-product inhibition of proline biosynthesis and of compartmentation in relation to stress-induced proline accumulation. Funct Plant Biol 3(4):513–525
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Burke JJ (1990) Variation among species in the temperature dependence of the reappearance of variable fluorescence following illumination. Plant Physiol 93:652–656
Cakmak I, Marschner H (1992) Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol 98(4):1222–1227
Chollet R, Vidal J, O’Leary MH (1996) Phosphoenolpyruvate carboxylase: a ubiquitous, highly regulated enzyme in plants. Annu Rev Plant Physiol Plant Mol Biol 47:273–298
Claussen W (2005) Proline as a measure of stress in tomato plants. Plant Sci 168(1):241–248
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16(6):735–743
Delauney AJ, Verma DPS (1993) Proline biosynthesis and osmoregulation in plants. Plant J 4(2):215–223
Demmig B, Winter K, Kruger A, Czygan FC (1987) Photoinhibition and zeaxanthin formation in intact leaves: a possible role of the xanthophyll cycle in the dissipation of excess light energy. Plant Physiol 84(2):218–224
Demmig-Adams B, Garab G, Adams W III, Govindjee (eds) (2014) Non photochemical quenching and energy dissipation in plants, algae and cyanobacteria. Advances in photosynthesis and respiration, vol 40. Springer, Dordrecht
Ding ZS, Zhao M, Jing YX, Li LB, Kuang TY (2007) Effect of overexpression of maize ppc gene on photosynthesis in transgenic rice plants. Acta Agron Sin 33:717–722 (in Chinese with English abstract)
Ding S, Lei M, Lu Q, Zhang A, Yin Y, Wen X, Zhang L, Lu C (2012a) Enhanced sensitivity and characterization of photosystem II in transgenic tobacco plants with decreased chloroplast glutathione reductase under chilling stress. Biochim Biophys Acta 1817(11):1979–1991
Ding ZS, Zhou BY, Sun XF, Zhao M (2012b) High light tolerance is enhanced by overexpressed PEPC in rice under drought stress. Acta Agron Sin 38(2):285–292
dos Santos CLV, Caldeira G (1999) Comparative responses of Helianthus annuus plants and calli exposed to NaCl: II. Growth rate and osmotic regulation in intact plants and calli. J Plant Physiol 155(6):769–777
Dutta S, Mohanty S, Tripathy BC (2009) Role of temperature stress on chloroplast biogenesis and protein import in pea. Plant Physiol 150(2):1050–1061
Eaton-Rye JJ, Tripathy BC, Sharkey TD (eds) (2012) Photosynthesis—plastid biology, energy conversion and carbon fixation. Advances in photosynthesis and respiration including bioenergy and related processes, vol 34. Springer, Dordrecht
Ehleringer JR, Sage RF, Flanagan LB, Pearcy RW (1991) Climate change and the evolution of C4 photosynthesis. Trends Ecol Evol 6(3):95–99
Ellsworth DS, Reich PB (1993) Canopy structure and vertical patterns of photosynthesis and related leaf traits in a deciduous forest. Oecologia 96:169–178
Fang LF, Ding ZS, Zhao M (2008) Characteristics of drought tolerance in ppc overexpressed rice seedlings. Acta Agron Sin 34:1220–1226
Fukayama H, Hatch MD, Tamai T, Tsuchida H, Sudoh S, Furbank RT, Miyao M (2003) Activity, regulation and physiological impacts of maize C(4)-specific phosphoenolpyruvate carboxylase overproduced in transgenic rice plants. Photosynth Res 77(2–3):227–239
Gegenheimer P (1990) Preparation of extracts from plants. Methods Enzymol 182:174–193
Gehlen J, Panstruga R, Smets H, Merkelbach S, Kleines M, Porsch P, Fladung M, Becker I, Rademacher T, Häusler RE, Hirsch HJ (1996) Effects of altered phosphoenolpyruvate carboxylase activities on transgenic C3 plant Solanum tuberosum. Plant Mol Biol 32(5):831–848
Gleeson D, Lelu-Walter M-A, Parkinson M (2005) Overproduction of proline in transgenic hybrid larch (Larix x leptoeuropaea (Dengler)) cultures renders them tolerant to cold, salt and frost. Mol Breed 15(1):21–29
Govindjee (1995) Sixty-three years since Kautsky: chlorophyll a fluorescence. Funct Plant Biol 22(2):131–160
Govindjee (2004) Chlorophyll a fluorescence: a bit of basics and history. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence: a probe of photosynthesis, advances in photosynthesis and respiration, vol 19. Springer, Dordrecht, pp 2–42
Govindjee, Amesz J, Fork DC (eds) (1986) Light emission by plants and bacteria. Academic Press Inc., Orlando
Gowik U, Westhoff P (2011) The path from C3 to C4 photosynthesis. Plant Physiol 155:56–63
Gururani MA, Venkatesh J, Ganesan M, Strasser RJ, Han Y, Kim J, Lee H-Y, Song P-S (2015) In Vivo assessment of cold tolerance through Chlorophyll-a fluorescence in transgenic zoysiagrass expressing mutant phytochrome A. PLoS One 10(5):e0127200. doi:10.1371/journal.pone.0127200
Haberlandt G (1904) Physiologische pflanzenanatomie, 3rd edn. W. Engelmann, Leipzig
Hamdani S, Qu M, Xin CP, Li Ming, Chu C, Govindjee, Zhu XG (2015) Variations between the photosynthetic properties of elite and landrace Chinese rice cultivars revealed by simultaneous measurements of 820 nm transmission signal and chlorophyll a fluorescence induction. J Plant Physiol 177:128–138
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51:463–499. doi:10.1146/annurev.arplant.51.1.463
Hatch MD (1987) C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultrastructure. Biochim Biophys Acta 895:81–106
Häusler RE, Kleines M, Uhrig H, Hirsch H-J, Smets H (1999) Overexpression of phosphoenolpyruvate carboxylase from Corynebacterium glutamicum lowers the CO2 compensation point (Γ*) and enhances dark and light respiration in transgenic potato. J Exp Bot 50(336):1231–1242
Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207(4):604–611
Horton P, Hague A (1988) Studies on the induction of chlorophyll fluorescence in isolated barley protoplasts. IV. Resolution of non-photochemical quenching. Biochim Biophys Acta 932:107–115
Horton P, Ruban AV, Walters RG (1996) Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol 47:655–684
Hudspeth RL, Grula JW, Dai Z, Edwards GE, Ku MS (1992) Expression of maize phosphoenolpyruvate carboxylase in transgenic tobacco: effects on biochemistry and physiology. Plant Physiol 98(2):458–464
Ishimaru K, Ichikawa H, Matsuoka M, Ohsugi R (1997) Analysis of a C4 maize pyruvate, orthophosphate dikinase, expressed in C3 transgenic Arabidopsis plants. Plant Sci 129:57–64
Ishimaru K, Ohkawa Y, Ishige T, Tobias DJ, Ohsugi R (1998) Elevated pyruvate, orthophosphate dikinase (PPDK) activity alters carbon metabolism in C3 transgenic potatoes with a C4 maize PPDK gene. Physiol Plant 103:340–346
Izui K, Matsumura H, Furumoto T, Kai Y (2004) Phosphoenolpyruvate carboxylase: a new era of structural biology. Annu Rev Plant Biol 55:69–84
Jiang CD, Wang X, Gao HY, Shi L, Chow WS (2011) Systemic regulation of leaf anatomical structure, photosynthetic performance, and high-light tolerance in sorghum. Plant Physiol 155(3):1416–1424
Jiao D, Huang X, Li X, Chi W, Kuang T, Zhang Q, Ku MB, Cho D (2002) Photosynthetic characteristics and tolerance to photo-oxidation of transgenic rice expressing C4 photosynthesis enzymes. Photosynth Res 72(1):85–93
Johnson MP, Davison PA, Ruban AV, Horton P (2008) The xanthophyll cycle pool size controls the kinetics of non-photochemical quenching in Arabidopsis thaliana. FEBS Lett 582(2):262–266
Karnovsky MJ (1965) A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J Cell Biol 27:137–138
Khedr AH, Abbas MA, Wahid AA, Quick WP, Abogadallah GM (2003) Proline induces the expression of salt-stress-responsive proteins and may improve the adaptation of Pancratium maritimum L. to salt-stress. J Exp Bot 54(392):2553–2562
Kirst H, Formighieri C, Melis A (2014) Maximizing photosynthetic efficiency and culture productivity in cyanobacteria upon minimizing the phycobilisome light-harvesting antenna size. Biochim BiophysActa 1837(10):1653–1664
Kogami H, Shono M, Koike T, Yanagisawa S, Izui K, Sentoku N, Tanifuji S, Uchimiya H, Toki S (1994) Molecular and physiological evaluation of transgenic tobacco plants expressing a maize phosphoenolpyruvate carboxylase gene under the control of the cauliflower mosaic virus 35S promoter. Transgenic Res 3(5):287–296
Krause GH, Weis E (1991) Chlorophyll fluorescence—the basics. Annu Rev Plant Physiol Plant Mol Biol 42:313–349
Ku MS, Agarie S, Nomura M, Fukayama H, Tsuchida H, Ono K, Hirose S, Toki S, Miyao M, Matsuoka M (1999) High-level expression of maize phosphoenolpyruvate carboxylase in transgenic rice plants. Nat Biotechnol 17(1):76–80
Lance C, Rustin P (1984) The central role of malate in plant metabolism. Physiol Veg 22:625–641
Latzko E, Kelly J (1983) The multi-faceted function of phosphoenolpyruvate carboxylase in C3 plants. Physiol Veg 21:805–815
Lipka V, Hausler RE, Radmacher T, Li J, Hirsch HJ, Kreuzaler F (1999) Solanum tuberosum double transgenic expression expressing phosphoenolpyruvate carboxylase and NADP-malic enzyme display reduced electron requirement for CO2 fixation. Plant Sci 144:93–105
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4):402–408
Long SP (1999) Environmental responses. In: Sage RF, Monson RK (eds) C4 plant biology. Academic Press, San Diego, pp 215–249. doi:10.1016/B978-012614440-6/50008-2
Madan S, Nainawatee HS, Jain RK, Chowdhury JB (1995) Proline and proline metabolising enzymes in in vitro selected NaCl-tolerant Brassica juncea L. under salt stress. Ann Bot (Lond) 76:51–57
Malkin S, Kok B (1966) Fluorescence induction studies in isolated chloroplasts. I. Number of components involved in the reaction and quantum yields. Biochim Biophys Acta 126:413–432
Masumoto C, Miyazawa S-I, Ohkawa H, Fukuda T, Taniguchi Y, Murayama S, Kusano M, Saito K, Fukayama H, Miyao M (2010) Phosphoenolpyruvate carboxylase intrinsically located in the chloroplast of rice plays a crucial role in ammonium assimilation. Proc Natl Acad Sci USA 107(11):5226–5231
Meimoun P, Gousset-Dupont A, Lebouteiller B, Ambard-Bretteville F, Besin E, Lelarge C, Mauve C, Hodges M, Vidal J (2009) The impact of PEPC phosphorylation on growth and development of Arabidopsis thaliana: molecular and physiological characterization of PEPC kinase mutants. FEBS Lett 583(10):1649–1652
Melzer E, O’Leary MH (1987) Anapleurotic CO2 fixation by phosphoenolpyruvate carboxylase in C3 plants. Plant Physiol 84(1):58–60
Misra PS, Mertz ET, Glover DV (1975) Studies on corn proteins: VIII. Free amino acid content of opaque-2 and double mutants. Cereal Chem 52:844–848
Miyao M, Fukayama H (2003) Metabolic consequences of overproduction of phosphoenolpyruvate carboxylase in C3 plants. Arch Biochem Biophys 414:197–203
Miyao M, Masumoto C, Miyazawa S, Fukayama H (2011) Lessons from engineering a single-cell C4 photosynthetic pathway into rice. J Exp Bot 62:3021–3029
Mohanty P, Govindjee, Wydrzynski T (1974) Salt-induced alterations of the fluorescence yield and emission spectra of Chlorella pyrenoidosa. Plant Cell Physiol 15(2):213–224
Müller P, Li XP, Niyogi KK (2001) Non-photochemical quenching: a response to excess light energy. Plant Physiol 125:1558–1566
Munday JC, Govindjee (1969a) Light-induced changes in the fluorescence yield of chlorophyll a in civo: III. The dip and the peak in the fluorescence transient of Chlorella pyrenoidosa. Biophys J 9(1):1–21
Munday JC, Govindjee (1969b) Light-induced changes in the fluorescence yield of chlorophyll a in civo: IV. The effect of preillumination on the fluorescence transient of Chlorella pyrenoidosa. Biophys J 9(1):22–35
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Env 25(2):239–250
Murchie EH, Niyogi KK (2011) Manipulation of photoprotection to improve plant photosynthesis. Plant Physiol 155:86–92
Nickrent DL (1994) From field to film: rapid sequencing methods for field-collected plant species. Biotechniques 16(3):470–475
Nilkens M, Kress E, Lambrev P, Miloslavina Y, Müller M, Holzwarth AR, Jahns P (2010) Identification of a slowly inducible zeaxanthin-dependent component of non-photochemical quenching of chlorophyll fluorescence generated under steady-state conditions in Arabidopsis. Biochim Biophys Acta 1797(4):466–475
Nimmo HG (2000) The regulation of phosphoenolpyruvate carboxylase in CAM plants. Trends Plant Sci 5(2):75–80
Niyogi KK (1999) Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol 50:333–359
Nobel PS (1976) Photosynthetic rates of sun versus shade leaves of Hyptis emoryi Torr. Plant Physiol 58:218–223
O’Leary MH (1982) Phosphoenolpyruvate carboxylase: an enzymologist’s view. Annu Rev Plant Physiol 33(1):297–315
O’Leary B, Park J, Plaxton William C (2011) The remarkable diversity of plant PEPC (phosphoenolpyruvate carboxylase): recent insights into the physiological functions and post-translational controls of non-photosynthetic PEPCs. Biochem J 436(1):15–34
Ort DR, Merchant SS, Alric J, Barkan A, Blankenship RE, Bock R, Croce R, Hanson MR, Hibberd JM, Long SP, Moore TA, Moroney J, Niyogi KK, Parry MA, Peralta-Yahya PP, Prince RC, Redding KE, Spalding MH, van Wijk KJ, Vermaas WF, von Caemmerer S, Weber AP, Yeates TO, Yuan JS, Zhu XG (2015) Redesigning photosynthesis to sustainability meet global food and bioenergy demand. Proc Natl Acad Sci USA 112(28):8529–8536
Osmond CB, Winter K, Ziegler H (1982) Functional significance of different pathways of CO2 fixation in photosynthesis. In: Lange OL, Noble PS, Osmond CB, Ziegler H (eds) Encyclopedia of plant physiology, new series, vol 12B. Springer, Berlin, pp 479–547
Outlaw WH, Du Z, Meng FX, Aghoram K, Riddle KA, Chollet R (2002) Requirements for activation of the signal-transduction network that leads to regulatory phosphorylation of leaf guard-cell phosphoenolpyruvate carboxylase during fusicoccin-stimulated stomatal opening. Arch Biochem Biophys 407:63–71
Oxborough K (2004) Imaging of chlorophyll a fluorescence: theoretical and practical aspects of an emerging technique for the monitoring of photosynthetic performance. J Exp Bot 55(400):1195–1205
Papageorgiou GC, Govindjee (eds) (2004) Chlorophyll a fluorescence: a signature of photosynthesis. Advances in photosynthesis and respiration, vol 19. Springer, Dordrecht
Pattanayak GK, Biswal AK, Reddy VS, Tripathy BC (2005) Light-dependent regulation of chlorophyll b biosynthesis in chlorophyllide a oxygenase overexpressing tobacco plants. Biochem Biophys Res Commun 326(2):466–471
Pereira WE, de Siqueira DL, Martínez CA, Puiatti M (2000) Gas exchange and chlorophyll fluorescence in four citrus rootstocks under aluminium stress. J Plant Physiol 157(5):513–520
Petrusa LM, Winicov I (1997) Proline status in salt tolerant and salt sensitive alfalfa cell lines and plants in response to NaCl. Plant Physiol Biochem 35:303–310
Plumley FG, Schmidt GW (1989) Nitrogen-dependent regulation of photosynthetic gene expression. Proc Natl Acad Sci USA 86(8):2678–2682
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975(3):384–394
Powles SB, Critchley C (1980) Effect of light intensity during growth on photoinhibition of intact attached bean leaflets. Plant Physiol 65:1181–1187
Putter J (1974) Peroxidase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Verlag Chemie, Weinhan, pp 685–690
Rademacher T, Hausler RE, Hirsch HJ, Zhang L, Lipka V, Weier D, Kreuzaler F, Peterhansel C (2002) An engineered phosphoenolpyruvate carboxylase redirects carbon and nitrogen flow in transgenic potato plants. Plant J 32(1):25–39
Raghavendra AS, Sage RF (eds) (2011) C4 photosynthesis and related CO2 concentrating mechanisms. Advances in photosynthesis and respiration, vol 32. Springer, Dordrecht
Raines CA (2011) Increasing photosynthetic carbon assimilation in C3 plants to improve crop yield: current and future strategies. Plant Physiol 155:36–42
Rhodes D, Handa S, Bressan RA (1986) Metabolic changes associated with adaptation of plant cells to water stress. Plant Physiol 82(4):890–903
Rose R, Rose CL, Omi SK, Forry KR, Durall DM, Bigg WL (1991) Starch determination by perchloric acid vs enzymes: evaluating the accuracy and precision of six colorimetric methods. J Agric Food Chem 39(1):2–11
Ruhil K, Sheeba, Ahmad A, Iqbal M, Tripathy BC (2015) Photosynthesis and growth responses of mustard (Brassica juncea L. cv Pusa Bold) plants to free air carbon dioxide enrichment (FACE). Protoplasma 252(4):935–946
Sage RF, Wedin DA, Li M (1999) The biogeography of C4 photosynthesis: patterns and controlling factors. In: Sage RF, Monson RK (eds) C4 plant biology. Academic Press, San Diego, pp 313–373
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, New York
Sanchez R, Flores A, Cejudo FJ (2006) Arabidopsis phosphoenolpyruvate carboxylase genes encode immunologically unrelated polypeptides and are differentially expressed in response to drought and salt stress. Planta 223(5):901–909
Santos CV (2004) Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Sci Hortic 103(1):93–99
Schreiber U (2004) Pulse-amplitude-modulation (PAM) fluorometry and saturation pulse method: an overview. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence: a signature of photosynthesis. Advances in photosynthesis and respiration, vol 19. Springer, Dordrecht, pp 279–319
Schreiber U, Armond PA (1978) Heat-induced changes of chlorophyll fluorescence in isolated chloroplasts and related heat-damage at the pigment level. Biochim Biophys Acta 502(1):138–151
Schreiber U, Bilger W, Neubauer C (1995) Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. In: Schulze E-D, Caldwell M (eds) Ecophysiology of photosynthesis, vol 100, Springer Study edn. Springer, Berlin, pp 49–70
Silva EN, Ribeiro RV, Ferreira-Silva SL, Viégas RA, Silveira JAG (2011) Salt stress induced damages on the photosynthesis of physic nut young plants. Scientia Agricola 68:62–68
Srivastava A, Guissé B, Greppin H, Strasser RJ (1997) Regulation of antenna structure and electron transport in PSII of Pisum sativum under elevated temperature probed by the fast polyphasic chlorophyll a fluorescence transient: OKJIP. Biochim Biophys Acta 1320:95–106
Stirbet A (2013) Excitonic connectivity between photosystem II units: what is it, and how to measure it? Photosynth Res 116:189–214
Stirbet A, Govindjee (2011) On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and Photosystem II: basics and applications of the OJIP fluorescence transient. J Photochem Photobiol B 104:236–257
Stirbet A, Govindjee (2012) Chlorophyll a fluorescence induction: understanding the thermal phase, the J-I-P rise. Photosynth Res 113:15–61
Stirbet A, Yu Riznichenko G, Rubin AB, Govindjee (2014) Modeling chlorophyll a fluorescence transient: relation to photosynthesis. Biochemistry (Moscow) 79:291–323
Strasser RJ, Srivastava A, Govindjee (1995) Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem Photobiol 61:32–42
Strasser RJ, Srivastava A, Tsimilli-Michael M (1999) Screening the vitality and photosynthetic activity of plants by fluorescence transient. In: Behl RK, Punia MS, Lather BPS (eds) Crop improvement for food security. SSARM, Hisar, pp 79–126
Strasser A, Srivastava A, Tsimilli-Michael M (2000) The fluorescence transient as a tool to characterize and screen photosynthetic samples. In: Yunus M, Pathre U, Mohanty P (eds) Probing photosynthesis: mechanisms, regulation and adaptation. Taylor & Francis, London, pp 445–483
Strasser R, Tsimilli-Michael M, Srivastava A (2004) Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence: a signature of photosynthesis, advances in photosynthesis and respiration, vol 19. Springer, Dordrecht, pp 321–362. doi:10.1007/978-1-4020-3218-9_12
Strasser RJ, Tsimilli-Michael M, Dangre D, Rai M (2007) Biophysical phenomics reveals functional building blocks of plants systems biology: a case study for the evaluation of the impact of mycorrhization with Piriformospora indica. In: Varma A, Oelmüler R (eds) Advanced techniques in soil microbiology. Soil Biology Series, vol 11. Springer, Heidelberg, pp 319–341
Sugiharto B, Sugiyama T (1992) Effects of nitrate and ammonium on gene expression of phosphoenolpyruvate carboxylase and nitrogen metabolism in maize leaf tissue during recovery from nitrogen stress. Plant Physiol 98(4):1403–1408
Suzuki S, Murai N, Burnell JN, Arai M (2000) Changes in photosynthetic carbon flow in transgenic rice plants that express C4-type phosphoenolpyruvate carboxykinase from Urochloa panicoides. Plant Physiol 124:163–172
Suzuki S, Murai N, Kasaoka K, Hiyoshi T, Imaseki H, Burnell JN, Arai M (2006) Carbon metabolism in transgenic rice plants that express phosphoenolpyruvate carboxylase and/or phosphoenolpyruvate carboxykinase. Plant Sci 170(5):1010–1019
Takeuchi Y, Akagi H, Kamasawa N, Osumi M, Honda H (2000) Aberrant chloroplasts in transgenic rice plants expressing a high level of maize NADP-dependent malic enzyme. Planta 211:265–274
Taniguchi Y, Ohkawa H, Masumoto C, Fukuda T, Tamai T, Lee K, Sudoh S, Tsuchida H, Sasaki H, Fukayama H, Miyao M (2008) Overproduction of C4 photosynthetic enzymes in transgenic rice plants: an approach to introduce the C4-like photosynthetic pathway into rice. J Exp Bot 59(7):1799–1809
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76(9):4350–4354
Tsimilli-Michael M, Strasser R (2008) In vivo assessment of stress impact on plant’s vitality: applications in detecting and evaluating the beneficial role of mycorrhization on host plants. In: Varma A (ed) mycorrhiza. Springer, Berlin, pp 679–703. doi:10.1007/978-3-540-78826-3_32
Tsimilli-Michael M, Eggenberg P, Biro B, Köves-Pechy K, Vörös I, Strasser RJ (2000) Synergistic and antagonistic effects of arbuscular mycorrhizal fungi and Azospirillum and Rhizobium nitrogen-fixers on the photosynthetic activity of alfalfa, probed by the polyphasic chlorophyll a fluorescence transient O-J-I-P. Appl Soil Ecol 15(2):169–182
Tsuchida H, Tamai T, Fukayama H, Agarie S, Nomura M, Onodera H, Ono K, Nishizawa Y, Lee BH, Hirose S, Toki S, Ku MSB, Matsuoka M, Miyao M (2001) High level expression of C4-specific NADP-malic enzyme in leaves and impairment of photoautotrophic growth in a C3 plant, rice. Plant Cell Physiol 42:138–145
Turan S, Tripathy BC (2015) Salt-stress induced modulation of chlorophyll biosynthesis during de-etiolation of rice seedlings. Physiol Plant 153(3):477–491
Vavasseur A, Raghavendra S (2005) Guard cell metabolism and CO2 sensing. New Physiol 165:665–682
Venekamp JH, Koot TM (1988) The sources of free proline and asparagine in field bean plants, Vicia faba L., during and after a short period of water withholding. J Plant Physiol 132(1):102–109
Vidal J, Chollet R (1997) Regulatory phosphorylation of C4 PEP carboxylase. Trends Plant Sci 2(6):230–237
von Caemmerer S, Farquhar GD (1981) Some relationships between the photochemistry and the gas exchange of leaves. Planta 153:376–387
Wang Y-M, Xu W-G, Hu L, Zhang L, Li Y, Du X-H (2012) Expression of maize gene encoding C4-pyruvate orthophosphate dikinase (PPDK) and C4-phosphoenolpyruvate carboxylase (PEPC) in transgenic Arabidopsis. Plant Mol Biol Rep 30(6):1367–1374
Wu XX, Ding HD, Chen JL, Zhang HJ, Zhu WM (2010) Attenuation of salt-induced changes in photosynthesis by exogenous nitric oxide in tomato (Lycopersicon esculentum Mill. L.) seedlings. Afr J Biotechnol 9:7837–7846
Younis ME, Hasaneen MNA, Tourky SMN (2009) Plant growth, metabolism and adaptation in relation to stress conditions. XXIV. Salinity biofertility interactive effects on proline, glycine and various antioxidants in Lactuca sativa. Plant. OMICS 2(5):197–205
Yusuf MA, Kumar D, Rajwanshi R, Strasser RJ, Tsimilli-Michael M, Govindjee, Sarin NB (2010) Overexpression of gamma-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviates abiotic stress: physiological and chlorophyll a fluorescence measurements. Biochim Biophys Acta 1797(8):1428–1438
Zhu X-G, Long SP, Ort DR (2008) What is the maximum efficiency with which photosynthesis can convert solar energy into biomass? Curr Opin Biotechnol 19(2):153–159
Acknowledgments
We thank Professor A.S. Raghavendra of the University of Hyderabad for providing us the anti-maize PEPC antibody. We also thank Kamal Ruhil and Barnali Padhi for their generous help in the experiments presented here. This work was supported by the National Agriculture Innovation Project (Grant No. NAIP/C4/C2043/2008-09 to BCT), from the Indian Council Agriculture Research, and Sir Jagdish Chandra Bose Fellowship to BCT. Govindjee thanks JNU for Visiting Professorship in its School of Life Sciences, and GIAN (Global Initiative on Academic Network, Ministry of Human Resources, Govt. of India) to lecture in the Spring of 2016 at JNU; he also thanks Rayme Dorsey (Plant Biology) for helping him with office work, and Jeff Haas and all the staff at Information Technology, Life Sciences, University of Illinois at Urbana-Champaign for helping him with both software and hardware related to the use of computers.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors honor George C. Papageourgiou, who was a good friend of Late Prasanna Mohanty, B. C. Tripathy’s mentor; both Papageorgiou and Mohanty were Ph.D. students of one of us (Govindjee).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kandoi, D., Mohanty, S., Govindjee et al. Towards efficient photosynthesis: overexpression of Zea mays phosphoenolpyruvate carboxylase in Arabidopsis thaliana . Photosynth Res 130, 47–72 (2016). https://doi.org/10.1007/s11120-016-0224-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-016-0224-3