Abstract
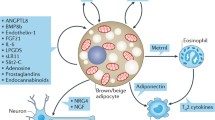
In recent years, brown adipose tissue (BAT) has been recognized not only as a main site of non-shivering thermogenesis in mammals, but also as an endocrine organ. BAT secretes a myriad of regulatory factors. These so-called batokines exert local autocrine and paracrine effects, as well as endocrine actions targeting tissues and organs at a distance. The endocrine batokines include peptide factors, such as fibroblast growth factor-21 (FGF21), neuregulin-4 (NRG4), phospholipid transfer protein (PLTP), interleukin-6, adiponectin and myostatin, and also lipids (lipokines; e.g., 12,13-dihydroxy-9Z-octadecenoic acid [12,13-diHOME]) and miRNAs (e.g., miR-99b). The liver, heart, and skeletal muscle are the most commonly reported targets of batokines. In response to BAT thermogenic activation, batokines such as NRG4 and PLTP are released and act to reduce hepatic steatosis and improve insulin sensitivity. Stress-induced interleukin-6-mediated signaling from BAT to liver favors hepatic glucose production through enhanced gluconeogenesis. Batokines may act on liver to induce the secretion of regulatory hepatokines (e.g. FGF21 and bile acids in response to miR-99b and PLTP, respectively), thereby resulting in a systemic expansion of BAT-originating signals. Batokines also target extrahepatic tissues: FGF21 and 12,13-diHOME are cardioprotective, whereas BAT-secreted myostatin and 12,13-diHOME influence skeletal muscle development and performance. Further research is needed to ascertain in humans the role of batokines, which have been identified mostly in experimental models. The endocrine role of BAT may explain the association between active BAT and a healthy metabolism in the human system, which is characterized by small amounts of BAT and a likely moderate BAT-mediated energy expenditure

Similar content being viewed by others
References
Cannon B. Nedergaard J Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359.
Ikeda K, Maretich P, Kajimura S. The Common and Distinct Features of Brown and Beige Adipocytes. Trends Endocrinol Metab. 2018;29:191–200.
Rasmussen AT. The glandular status of multilocular brown adipose tissue. Endocrinology. 1922;6:760–70.
Betz MJ, Enerbäck S. Human Brown Adipose Tissue: What We Have Learned So Far. Diabetes. 2015;64:2352–60.
Villarroya F, Cereijo R, Villarroya J, Giralt M. Brown adipose tissue as a secretory organ. Nat Rev Endocrinol. 2017;13:26–35.
Villarroya F, Gavaldà-Navarro A, Peyrou M, Villarroya J, Giralt M. The lives and times of brown adipokines. Trends Endocrinol Metab. 2017;28:855–67.
Villarroya J, Cereijo R, Gavaldà-Navarro A, Peyrou M, Giralt M, Villarroya F. New insights into the secretory functions of brown adipose tissue. J Endocrinol. 2019;243:R19–27.
White A, Levine R. History of Hormones. In: Goldberger RF, Yamamoto KR, editors. Biological Regulation and Development. New York: Springer Science+Business Media; 1982. p. 1–24.
Horwitz BA, Inokuchi T, Moore BJ, Stern JS. The effect of brown fat removal on the development of obesity in Zucker and Osborne-Mendel rats. Int J Obes. 1985;9(Suppl 2):43–8.
Rothwell NJ, Stock MJ. Surgical removal of brown fat results in rapid and complete compensation by other depots. Am J Physiol. 1989;257:R253-258.
Grunewald ZI, Winn NC, Gastecki ML, Woodford ML, Ball JR, Hansen SA, Sacks HS, Vieira-Potter VJ, Padilla J. Removal of interscapular brown adipose tissue increases aortic stiffness despite normal systemic glucose metabolism in mice. Am J Physiol Regul Integr Comp Physiol. 2018;314:R584-R597.
Stern JS, Inokuchi T, Castonguay TW, Wickler SJ, Horwitz BA. Scapular brown fat removal enhances development of adiposity in cold-exposed obese Zucker rats. Am J Physiol. 1984;247:R918–26.
Moore BJ, Inokuchi T, Stern JS, Horwitz BA. Brown adipose tissue lipectomy leads to increased fat deposition in Osborne-Mendel rats. Am J Physiol. 1985;248:R231–5.
Kong X, Yao T, Zhou P, Kazak L, Tenen D, Lyubetskaya A, Dawes BA, Tsai L, Kahn BB, Spiegelman BM, Liu T, Rosen ED. Brown adipose tissue controls skeletal muscle function via the secretion of myostatin. Cell Metab. 2018;28:631–43.
Stanford KI, Lynes MD, Takahashi H, Baer LA, Arts PJ, May FJ, Lehnig AC, Middelbeek RJW, Richard JJ, So K, Chen EY, Gao F, Narain NR, Distefano G, Shettigar VK, Hirshman MF, Ziolo MT, Kiebish MA, Tseng YH, Coen PM, Goodyear LJ. 12,13-diHOME: An Exercise-Induced Lipokine that Increases Skeletal Muscle Fatty Acid Uptake. Cell Metab. 2018;27:1111–20.
Lowell BB, Susulic V, Hamann A, Lawitts JA, Himms-Hagen J, Boyer BB, Kozak LP, Flier JS. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993;366:740–2.
Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper M-E, Kozak LP. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387:90–4.
Wang Y, Paulo E, Wu D, Wu Y, Huang W, Chawla A, Wang B. Adipocyte Liver Kinase b1 Suppresses Beige Adipocyte Renaissance Through Class IIa Histone Deacetylase 4. Diabetes. 2017;66:2952–63.
Villarroya F, Giralt M. The beneficial effects of brown fat transplantation: further evidence of an endocrine role of brown adipose tissue. Endocrinology. 2015;156:2368–70.
White JD, Dewal RS, Stanford KI. The beneficial effects of brown adipose tissue transplantation. Mol Aspects Med. 2019;68:74–81.
Gunawardana SC. Therapeutic value of brown adipose tissue: Correcting metabolic disease through generating healthy fat. Adipocyte. 2012;1:250–5.
Payab M, Abedi M, Foroughi Heravani N, Hadavandkhani M, Arabi M, Tayanloo-Beik A, Sheikh Hosseini M, Gerami H, Khatami F, Larijani B, Abdollahi M, Arjmand B. Brown adipose tissue transplantation as a novel alternative to obesity treatment: a systematic review. Int J Obes (Lond). 2021;45:109–21.
Yuan X, Hu T, Zhao H, Huang Y, Ye R, Lin J, Zhang C, Zhang H, Wei G, Zhou H, Dong M, Zhao J, Wang H, Liu Q, Lee HJ, Jin W, Chen ZJ. Brown adipose tissue transplantation ameliorates polycystic ovary syndrome. Proc Natl Acad Sci U S A. 2016;113:2708–13.
Du L, Wang Y, Li CR, Chen LJ, Cai JY, Xia ZR, Zeng WT, Wang ZB, Chen XC, Hu F, Zhang D, Xing XW, Yang ZX. Rat BAT xenotransplantation recovers the fertility and metabolic health of PCOS mice. J Endocrinol. 2020 Dec 1:JOE-20–0068.R1. https://doi.org/10.1530/JOE-20-0068. Online ahead of print.
Thomou T, Mori MA, Dreyfuss JM, Konishi M, Sakaguchi M, Wolfrum C, Rao TN, Winnay JN, Garcia-Martin R, Grinspoon SK, Gorden P, Kahn CR. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature. 2017;542:450–5.
Pinckard KM, Shettigar VK, Wright KR, Abay E, Baer LA, Vidal P, Dewal RS, Das D, Duarte-Sanmiguel S, Hernández-Saavedra D, Arts PJ, Lehnig AC, Bussberg V, Narain NR, Kiebish MA, Yi F, Sparks LM, Goodpaster BH, Smith SR, Pratley RE, Lewandowski ED, Raman SV, Wold LE, Gallego-Perez D, Coen PM, Ziolo MT, Stanford K. A Novel Endocrine Role the BAT-Released Lipokine 12,13-diHOME to Mediate Cardiac Function. Circulation. 2021;143:145–59.
Hondares E, Iglesias R, Giralt A, Gonzalez FJ, Giralt M, Mampel T, Villarroya F. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J Biol Chem. 2011;286:12983–90.
Giralt M, Gavaldà-Navarro A, Villarroya F. Fibroblast growth factor-21, energy balance and obesity. Mol Cell Endocrinol. 2015;418(Pt 1):66–73.
Zarei M, Pizarro-Delgado J, Barroso E, Palomer X, Vázquez-Carrera M. Targeting FGF21 for the Treatment of Nonalcoholic Steatohepatitis. Trends Pharmacol Sci. 2020;41:199–208.
Kliewer SA, Mangelsdorf DJ. A Dozen Years of Discovery: Insights into the Physiology and Pharmacology of FGF21. Cell Metab. 2019;29:246–53.
Markan KR, Naber MC, Ameka MK, Anderegg MD, Mangelsdorf DJ, Kliewer SA, Mohammadi M, Potthoff MJ. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes. 2014;63:4057–63.
Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng YH, Goodyear LJ. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013;123:215–23.
Keipert S, Kutschke M, Lamp D, Brachthäuser L, Neff F, Meyer CW, Oelkrug R, Kharitonenkov A, Jastroch M. Genetic disruption of uncoupling protein 1 in mice renders brown adipose tissue a significant source of FGF21 secretion. Mol Metab. 2015;4:537–42.
Wang GX, Zhao XY, Meng ZX, Kern M, Dietrich A, Chen Z, Cozacov Z, Zhou D, Okunade AL, Su X, Li S, Blüher M, Lin JD. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat Med. 2014;20:1436–1443.
Christian M. Transcriptional fingerprinting of “browning” white fat identifies NRG4 as a novel adipokine. Adipocyte. 2014;4:50–4.
Chen Y, Buyel JJ, Hanssen MJ, Siegel F, Pan R, Naumann J, Schell M, van der Lans A, Schlein C, Froehlich H, Heeren J, Virtanen KA, van Marken LW, Pfeifer A. Exosomal microRNA miR-92a concentration in serum reflects human brown fat activity. Nat Commun. 2016;7:11420.
Sponton CH, Hosono T, Taura J, Jedrychowski MP, Yoneshiro T, Wang Q, Takahashi M, Matsui Y, Ikeda K, Oguri Y, Tajima K, Shinoda K, Pradhan RN, Chen Y, Brown Z, Roberts LS, Ward CC, Taoka H, Yokoyama Y, Watanabe M, Karasawa H, Nomura DK, Kajimura S. The regulation of glucose and lipid homeostasis via PLTP as a mediator of BAT-liver communication. EMBO Rep. 2020;21:e49828.
Qing H, Desrouleaux R, Israni-Winger K, Mineur YS, Fogelman N, Zhang C, Rashed S, Palm NW, Sinha R, Picciotto MR, Perry RJ, Wang A. Origin and Function of Stress-Induced IL-6 in Murine Models. Cell. 2020;182:372–87.
Burýsek L, Houstek J. beta-Adrenergic stimulation of interleukin-1alpha and interleukin-6 expression in mouse brown adipocytes. FEBS Lett. 1997;411:83–6.
Mauer J, Chaurasia B, Goldau J, Vogt MC, Ruud J, Nguyen KD, Theurich S, Hausen AC, Schmitz J, Brönneke HS, Estevez E, Allen TL, Mesaros A, Partridge L, Febbraio MA, Chawla A, Wunderlich FT, Brüning JC. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat Immunol. 2014;15:423–30.
Villarroya F, Cereijo R, Villarroya J, Gavaldà-Navarro A, Giralt M. Toward an Understanding of How Immune Cells Control Brown and Beige Adipobiology. Cell Metab. 2018;27:954–961.
Shen H, Jiang L, Lin JD, Omary MB, Rui L. Brown fat activation mitigates alcohol-induced liver steatosis and injury in mice. J Clin Invest. 2019;129:2305–17.
Min SY, Kady J, Nam M, Rojas-Rodriguez R, Berkenwald A, Kim JH, Noh HL, Kim JK, Cooper MP, Fitzgibbons T, Brehm MA, Corvera S. Human “brite/beige” adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice. Nat Med. 2016;22:312–8.
Liu D, Li Y, Shang Y, Wang W, Chen SZ. Effect of brown adipose tissue/cells on the growth of mouse hepatocellular carcinoma in vitro and in vivo. Oncol Lett. 2019;17:3203–10.
Yilmaz Y, Ones T, Purnak T, Ozguven S, Kurt R, Atug O, Turoglu HT, Imeryuz N. Association between the presence of brown adipose tissue and non-alcoholic fatty liver disease in adult humans. Aliment Pharmacol Ther. 2011;34:318–323.
Duncan JG, Fong JL, Medeiros DM, Finck BN, Kelly DP. Insulin-resistant heart exhibits a mitochondrial biogenic response driven by the peroxisome proliferator-activated receptor-alpha/PGC-1alpha gene regulatory pathway. Circulation. 2007;115:909–17.
Ilkun O, Wilde N, Tuinei J, Pires KM, Zhu Y, Bugger H, Soto J, Wayment B, Olsen C, Litwin SE, Abel DE. Antioxidant treatment normalizes mitochondrial energetics and myocardial insulin sensitivity independently of changes in systemic metabolic homeostasis in a mouse model of the metabolic syndrome. J Mol Cell Cardiol. 2015;85:104–16.
Thoonen R, Ernande L, Cheng J, Nagasaka Y, Yao V, Miranda-Bezerra A, Chen C, Chao W, Panagia M, Sosnovik DE, Puppala D, Armoundas AA, Hindle A, Bloch KD, Buys ES, Scherrer-Crosbie M. Functional brown adipose tissue limits cardiomyocyte injury and adverse remodeling in catecholamine-induced cardiomyopathy. J Mol Cell Cardiol. 2015;84:202–11.
Zhou X, Li Z, Qi M, Zhao P, Duan Y, Yang G, Yuan L. Brown adipose tissue-derived exosomes mitigate the metabolic syndrome in high fat diet mice. Theranostics. 2020;10:8197–210.
Planavila A, Redondo I, Hondares E, Vinciguerra M, Munts C, Iglesias R, Gabrielli LA, Sitges M, Giralt M, van Bilsen M, Villarroya F. Fibroblast growth factor 21 protects against cardiac hypertrophy in mice. Nat Commun. 2013;4:2019.
Ruan CC, Kong LR, Chen XH, Ma Y, Pan XX, Zhang ZB, Gao PJ. A2A receptor activation attenuates hypertensive cardiac remodeling via promoting brown adipose tissue-derived FGF21. Cell Metab. 2018;28:476–89.
Iacobellis G, Bianco AC. Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends Endocrinol Metab. 2011;22:450–7.
Chechi K, Vijay J, Voisine P, Mathieu P, Bossé Y, Tchernof A, Grundberg E, Richard D. UCP1 expression-associated gene signatures of human epicardial adipose tissue. JCI Insight. 2019;4:e123618.
Rodriguez J, Vernus B, Chelh I, Cassar-Malek I, Gabillard JC, Hadj Sassi A, Seiliez I, Picard B, Bonnieu A. Myostatin and the skeletal muscle atrophy and hypertrophy signaling pathways. Cell Mol Life Sci. 2014;71:4361–71.
Shan T, Liang X, Bi P, Kuang S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1α-Fndc5 pathway in muscle. FASEB J. 2013;27:1981–9.
Steculorum SM, Ruud J, Karakasilioti I, Backes H, Engström Ruud L, Timper K, Hess ME, Tsaousidou E, Mauer J, Vogt MC, Paeger L, Bremser S, Klein AC, Morgan DA, Frommolt P, Brinkkötter PT, Hammerschmidt P, Benzing T, Rahmouni K, Wunderlich FT, Kloppenburg P, Brüning JC. AgRP Neurons Control Systemic Insulin Sensitivity via Myostatin Expression in Brown Adipose Tissue. Cell. 2016;165:125–38.
Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Højlund K, Gygi SP, Spiegelman BM. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–8.
Cereijo R, Gavaldà-Navarro A, Cairó M, Quesada-López T, Villarroya J, Morón-Ros S, Sánchez-Infantes D, Peyrou M, Iglesias R, Mampel T, Turatsinze JV, Eizirik DL, Giralt M, Villarroya F. CXCL14, a Brown Adipokine that Mediates Brown-Fat-to-Macrophage Communication in Thermogenic Adaptation. Cell Metab. 2018;28:750–63.
Campderros L, Moure R, Cairó M, Gavaldà-Navarro A, Quesada-López T, Cereijo R, Giralt M, Villarroya J, Villarroya F. Brown adipocytes secrete GDF15 in response to thermogenic activation. Obesity (Silver Spring). 2019;27:1606–16.
Villarroya F, Cereijo R, Gavaldà-Navarro A, Villarroya J, Giralt M. Inflammation of brown/beige adipose tissues in obesity and metabolic disease. J Intern Med. 2018;284:492–504.
Henningsen JB, Scheele C. Brown Adipose Tissue: A Metabolic Regulator in a Hypothalamic Cross Talk? Annu Rev Physiol. 2021;83:279–301.
Néchad M, Ruka E, Thibault J. Production of nerve growth factor by brown fat in culture: relation with the in vivo developmental stage of the tissue. Comp Biochem Physiol Comp Physiol. 1994;107:381–8.
Nisoli E, Tonello C, Benarese M, Liberini P, Carruba MO. Expression of nerve growth factor in brown adipose tissue: implications for thermogenesis and obesity. Endocrinology. 1996;137:495–503.
Hu B, Jin C, Zeng X, Resch JM, Jedrychowski MP, Yang Z, Desai BN, Banks AS, Lowell BB, Mathis D, Spiegelman BM. γδ T cells and adipocyte IL-17RC control fat innervation and thermogenesis. Nature. 2020;578:610–4.
Zeng X, Ye M, Resch JM, Jedrychowski MP, Hu B, Lowell BB, Ginty DD, Spiegelman BM. Innervation of thermogenic adipose tissue via a calsyntenin 3beta-S100b axis. Nature. 2019;569:229–35.
Becher T, Palanisamy S, Kramer DJ, Eljalby M, Marx SJ, Wibmer AG, Butler SD, Jiang CS, Vaughan R, Schöder H, Mark A, Cohen P. Brown adipose tissue is associated with cardiometabolic health. Nat Med. 2021;27:58–65.
Cohen P, Levy JD, Zhang Y, Frontini A, Kolodin DP, Svensson KJ, Lo JC, Zeng X, Ye L, Khandekar MJ, Wu J, Gunawardana SC, Banks AS, Camporez JP, Jurczak MJ, Kajimura S, Piston DW, Mathis D, Cinti S, Shulman GI, Seale P, Spiegelman BM. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 2014;156:304–316.
Becher, Palanisamy S, Kramer DJ, Marx SJ, Wibmer AG, Del Gaudio I, Butler SD, Jiang CS, Vaughan R, Schöder H, Di Lorenzo A, Mark A, Cohen P. Brown Adipose Tissue is Associated with Improved Cardiometabolic Health and Regulates Blood Pressure. bioRxiv 2020;53351880. https://doi.org/10.1101/2020.02.08.933754.
Fitzgibbons TP, Kogan S, Aouadi M, Hendricks GM, Straubhaar J, Czech MP. Similarity of mouse perivascular and brown adipose tissues and their resistance to diet-induced inflammation. Am J Physiol Heart Circ Physiol. 2011;301:H1425–37.
Tonello C, Giordano A, Cozzi V, Cinti S, Stock MJ, Carruba MO, Nisoli E. Role of sympathetic activity in controlling the expression of vascular endothelial growth factor in brown fat cells of lean and genetically obese rats. FEBS Lett. 1999;442:167–72.
Sun K, Kusminski CM, Luby-Phelps K, Spurgin SB, An YA, Wang QA, Holland WL, Scherer PE. Brown adipose tissue derived VEGF-A modulates cold tolerance and energy expenditure. Mol Metab. 2014;3:474–83.
Pellegrinelli V, Peirce VJ, Howard L, S, Türei D, Senzacqua M, Frontini A, Dalley JW, Horton AR, Bidault G, Severi I, Whittle A, Rahmouni K, Saez-Rodriguez J, Cinti S, Davies AM, Vidal-Puig A. Adipocyte-secreted BMP8b mediates adrenergic-induced remodeling of the neuro-vascular network in adipose tissue. Nat Commun. 2018;9:4974.
Wang CH, Lundh M, Fu A, Kriszt R, Huang TL, Lynes MD, Leiria LO, Shamsi F, Darcy J, Greenwood BP, Narain NR, Tolstikov V, Smith KL, Emanuelli B, Chang YT, Hagen S, Danial NN, Kiebish MA, Tseng YH. CRISPR-engineered human brown-like adipocytes prevent diet-induced obesity and ameliorate metabolic syndrome in mice. Sci Transl Med. 2020;12(558):eaaz8664
Deshmukh AS, Peijs L, Beaudry JL, Jespersen NZ, Nielsen CH, Ma T, Brunner AD, Larsen TJ, Bayarri-Olmos R, Prabhakar BS, Helgstrand C, Severinsen MCK, Holst B, Kjaer A, Tang-Christensen M, Sanfridson A, Garred P, Privé GG, Pedersen BK, Gerhart-Hines Z, Nielsen S, Drucker DJ, Mann M, Scheele C. Proteomics-Based Comparative Mapping of the Secretomes of Human Brown and White Adipocytes Reveals EPDR1 as a Novel Batokine. Cell Metab. 2019;30:963–75.
Hondares E, Gallego-Escuredo JM, Flachs P, Frontini A, Cereijo R, Goday A, Perugini J, Kopecky P, Giralt M, Cinti S, Kopecky J, Villarroya F. Fibroblast growth factor-21 is expressed in neonatal and pheochromocytoma-induced adult human brown adipose tissue. Metabolism. 2014;63:312–7.
Tutunchi H, Ostadrahimi A, Hosseinzadeh-Attar MJ, Miryan M, Mobasseri M, Ebrahimi-Mameghani M. A systematic review of the association of neuregulin 4, a brown fat-enriched secreted factor, with obesity and related metabolic disturbances. Obes Rev. 2020;21(2):e12952
Sookoian S, Pirola CJ. Nonalcoholic Fatty Liver Disease Progresses into Severe NASH when Physiological Mechanisms of Tissue Homeostasis Collapse. Ann Hepatol. 2018;17:182–186.
Chen LL, Peng MM, Zhang JY, Hu X, Min J, Huang QL, Wan LM. Elevated circulating Neuregulin4 level in patients with diabetes. Diabetes Metab Res Rev. 2017;33(4).
Vasan SK, Noordam R, Gowri MS, Neville MJ, Karpe F, Christodoulides C. The proposed systemic thermogenic metabolites succinate and 12,13-diHOME are inversely associated with adiposity and related metabolic traits: evidence from a large human cross-sectional study. Diabetologia. 2019;62:2079–87.
R Cereijo T Quesada-López A Gavaldà-Navarro J Tarasco S Pellitero M Reyes M Puig-Domingo M Giralt D Sanchez-Infantes F Villarroya 2020 The chemokine CXCL14 is negatively associated with obesity and concomitant type 2 diabetes in humans. Int J Obes 2021. https://doi.org/10.1038/s41366-020-00732-y.
Cereijo R, Taxerås SD, Piquer-Garcia I, Pellitero S, Martínez E, Tarascó J, Moreno P, Balibrea J, Puig-Domingo M, Jiménez-Pavón D, Lerin C, Villarroya F, Sánchez-Infantes D. Elevated Levels of Circulating miR-92a Are Associated with Impaired Glucose Homeostasis in Patients with Obesity and Correlate with Metabolic Status After Bariatric Surgery. Obes Surg. 2020;30:174–9.
Lidell ME. Brown Adipose Tissue in Human Infants. Handb Exp Pharmacol. 2019;251:107–23.
Sánchez-Infantes D, Gallego-Escuredo JM, Díaz M, Aragonés G, Sebastiani G, López-Bermejo A, de Zegher F, Domingo P, Villarroya F, Ibáñez L. Circulating FGF19 and FGF21 surge in early infancy from infra- to supra-adult concentrations. Int J Obes (Lond). 2015;39:742–6.
Whittle AJ, Carobbio S, Martins L, Slawik M, Hondares E, Vázquez MJ, Morgan D, Csikasz RI, Gallego R, Rodriguez-Cuenca S, Dale M, Virtue S, Villarroya F, Cannon B, Rahmouni K, López M, Vidal-Puig A. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. 2012;149:871–85.
Heaton JM. The distribution of brown adipose tissue in the human. J Anat. 1972;112(Pt 1):35–9.
Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–17.
Steinberg JD, Vogel W, Vegt E. Factors influencing brown fat activation in FDG PET/CT: a retrospective analysis of 15,000+ cases. Br J Radiol. 90:20170093
Adamczak M, Rzepka E, Chudek J, Wiecek A. Ageing and plasma adiponectin concentration in apparently healthy males and females. Clin Endocrinol (Oxf). 2005;62:114–8.
Hanks LJ, Gutiérrez OM, Bamman MM, Ashraf A, McCormick KL, Casazza K. Circulating levels of fibroblast growth factor-21 increase with age independently of body composition indices among healthy individuals. J Clin Transl Endocrinol. 2015;2:77–82
Villarroya J, Gallego-Escuredo JM, Delgado-Anglés A, Cairó M, Moure R, Gracia Mateo M, Domingo JC, Domingo P, Giralt M, Villarroya F. Aging is associated with increased FGF21 levels but unaltered FGF21 responsiveness in adipose tissue. Aging Cell. 2018;17:e12822.
Petruzzelli M, Schweiger M, Schreiber R, Campos-Olivas R, Tsoli M, Allen J, Swarbrick M, Rose-John S, Rincon M, Robertson G, Zechner R, Wagner EF. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 2014;20:433–447.
Abdullahi A, Samadi O, Auger C, Kanagalingam T, Boehning D, Bi S, Jeschke MG. Browning of white adipose tissue after a burn injury promotes hepatic steatosis and dysfunction. Cell Death Dis. 2019;10:870.
Moulder R, Bhosale SD, Goodlett DR, Lahesmaa R. Analysis of the plasma proteome using iTRAQ and TMT-based Isobaric labeling. Mass Spectrom Rev. 2018;37:583–606.
Armingol E, Officer A, Harismendy O, Lewis NE. Deciphering cell-cell interactions and communication from gene expression. Nat Rev Genet. 2020;9:1–18.
Funding
Supported by Ministerio de Ciencia, Innovación y Universidades, and Agencia Estatal de Investigación (Spain); and Fondo Europeo de Desarollo Regional, EU (grant SAF2017-85722-R) and Instituto de Salud Carlos III (grants PI17-00420 and PI20-00,106).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
No primary experimental or clinical data are included in the manuscript, and, accordingly, no ethics approval is required.
Consent to participate
No primary clinical data are included in the manuscript and, accordingly, no consent to participate statements are needed.
Consent for publication
All co-authors participated in the elaboration of the manuscript, approved the final version to be submitted and consent for publication. (include appropriate statements).
Conflicts of interest
There is no conflicts of interests in relation to this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gavaldà-Navarro, A., Villarroya, J., Cereijo, R. et al. The endocrine role of brown adipose tissue: An update on actors and actions. Rev Endocr Metab Disord 23, 31–41 (2022). https://doi.org/10.1007/s11154-021-09640-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-021-09640-6