Abstract
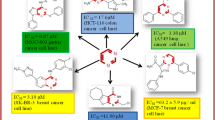
N′-((2-Chloroquinolin-3-yl)methylene)-2-cyanoacetohydrazide (3) was synthesized then treated with aromatic aldehydes in basic medium to afford the arylidene derivatives 4a–e. Reaction of 4a–c with hydrazine hydrate in boiling ethanol gave the 3-aminopyrazoles 5a–c. Base promoted Michael addition of 3 to arylidene malononitriles 6 afforded 2-pyridones 9a–d. Cyclocondensation of 3 with some salicylaldehyde derivatives gave the iminocoumarins 10a–c; these underwent acid-catalyzed hydrolysis to give coumarins 11a–c. Coupling of 3 with arene diazonium chloride in pyridine afforded the arylhydrazononitriles 12a–c. Heterocyclization of 12a with formalin and piperidine in warm ethanol gave the 1,2,4-triazine derivative 13. The mechanisms and the chemoselectively of these reactions are discussed. The newly synthesized compounds were tested for antibacterial and anticancer activity. Pyridone 9b and coumarin 11c had the most potent antibacterial activity against S. aureus. Acrylamide 4d, pyridones 9a, c, and 1,2,4-triazine 13 were the most active anticancer compounds, with a broad range of activity against most of the tumor cell lines tested.







Similar content being viewed by others
References
N.C. Desai, K.M. Rajpara, V.V. Joshi, Bioorg. Med. Chem. Lett. 22, 6871–6875 (2012)
S. Tabassum, T.H. Suresha Kumara, J.P. Jasinski, S.P. Millikan, H.S. Yathirajan, P.S. Sujan Ganapathy, H.B.V. Sowmya, S.S. More, G. Nagendrappa, M. Kaur, G. Jose, J. Mol. Str. 1070, 10–20 (2014)
P. Nasveld, S. Kitchener, Trans. R. Soc. Trop. Med. Hyg. 99, 2–5 (2005)
J.E. Charris, G.M. Lobo, J. Camacho, R. Ferrer, A. Barazarte, J.N. Dominguez, N. Gamboa, J.R. Rodrigues, J.E. Angel, Lett. Drug Des. Discov. 4, 49–54 (2007)
V.V. Kouznetsov, L.Y.V. Méndez, A.M. Acevedo, Lett. Drug Des. Discov. 7, 710–715 (2010)
Y.-L. Chen, I.-L. Chen, C.-M. Lu, C.-C. Tzeng, L.-T. Tsao, J.-P. Wang, Bioorg. Med. Chem. 12, 387–392 (2004)
R.E. Khidre, B.F. Abdel-Wahab, F.A. Badria, Lett. Drug Des. Discov. 8, 640–648 (2011)
V.V. Kouznetsov, L.Y.V. Méndez, S.M. Leal, U.M. Cruz, C.A. Coronado, C.M.M. Gómez, A.R.R. Bohórquez, P.E. Rivero, Lett. Drug Des. Discov. 4, 293–296 (2007)
M.P. Maguire, K.R. Sheets, K. McVety, A.P. Spada, A. Zillberstein, J. Med. Chem. 37, 2129–2137 (1994)
S. Bajare, J. Anthony, A. Nair, R. Marita, A. Damre, D. Patel, C. Rao, H. Sivaramakrishnan, N. Deka, Eur. J. Med. Chem. 58, 355–360 (2012)
B.F. Abdel-Wahab, R.E. Khidre, A.A. Farahat, A.-A.S. El-AhI, Arkivoc 1, 211–276 (2012)
N.C. Desai, N.R. Shihory, G.M. Kotadiya, Lett. Drug Design Discov. 10, 632–639 (2013)
B.M. Mistry, S. Jauhari, Med. Chem. Res. 22, 647–658 (2013)
S. Bondock, W. Khalifa, A.A. Fadda, Eur. J. Med. Chem. 42, 948–954 (2007)
S. Bondock, R. Rabie, H.A. Etman, A.A. Fadda, Eur. J. Med. Chem. 43, 2122–2129 (2008)
S. Bondock, W. Fadaly, M.A. Metwally, Eur. J. Med. Chem. 44, 4813–4818 (2009)
S. Bondock, W. Fadaly, M.A. Metwally, Eur. J. Med. Chem. 45, 3692–3701 (2010)
S. Bondock, W. Khalifa, A.A. Fadda, Eur. J. Med. Chem. 46, 2555–2561 (2011)
S. Bondock, T. Naser, Y.A. Ammar, Eur. J. Med. Chem. 62, 270–279 (2013)
T. Nasr, S. Bondock, S. Eid, Eur. J. Med. Chem. 84, 491–504 (2014)
S. Bondock, Res. Chem. Intermed. (in press) (2014). doi.10.1007/s11164-014-1672-z
S. Bondock, S. Adel, H.A. Etman, F.A. Fadria, Eur. J. Med. Chem. 48, 192–199 (2012)
T. Nasr, S. Bondock, M. Youns, Eur. J. Med. Chem. 76, 539–548 (2014)
O. Meth-Cohn, B. Narine, B. Tarnowski, J. Chem. Soc. Perkin Trans 1, 1520–1530 (1981)
H.I. Adamali, T.M. Maher, Drug Des. Dev. Ther. 6, 261–272 (2012)
I.V. Dyachenko, V.D. Dyachenko, E.B. Rusanov, Russ. J. Org. Chem. 43, 83–89 (2007)
J.G. Schantt, P. Hebeisen, Tetrahedron 46, 395–406 (1990)
A.S. Shawali, M.A. El-Galil, Tetrahedron 27, 4305–4316 (1971)
A.H. Shamroukh, M.E.A. Zaki, E.M.H. Morsy, F.M. Abdel-Motti, F.M.E. Abdel-Megeid, Arch. Pharm. Chem. Life Sci. 340, 345–351 (2007)
P. Skehan, R. Storeng, D. Scudiero, A. Monks, J. McMahon, D. Vistica, J.T. Warren, H. Bokesch, S. Kenny, M.R. Boyd, J. Natl Cancer Inst. 82, 1107–1112 (1990)
A. Monks, D. Scudiero, P. Skehan, R. Shoemaker, K. Paul, D. Vistica, C. Hose, J. Langley, P. Cronise, A. Vaigro-Wolff, M. Gray-Goodr, H. Campbell, J. Mayo, J.M. Boyd, J. Natl Cancer Inst. 83, 757–766 (1991)
Acknowledgments
We express our appreciation to the King Abd-Aziz City for Science and Technology (KACST), Saudi Arabia, for financial support of this work (project no. P-S-12-0015).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bondock, S., Gieman, H. Synthesis, antibacterial and anticancer evaluation of some new 2-chloro-3-hetarylquinolines. Res Chem Intermed 41, 8381–8403 (2015). https://doi.org/10.1007/s11164-014-1899-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-014-1899-8