Abstract
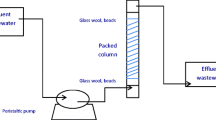
In this study, the adsorptive effectiveness of sustainable and cost-effective eucalyptus bark biomass in the removal of methylene blue (MB) dye from its aqueous solution has been tested using a packed bed up-flow column experiment. A series of column experiments using raw eucalyptus bark was performed to determine the breakthrough curves with varying inlet MB dye flow rate (10–15 mL min−1), initial MB dye concentration (50–100 mg L−1) and adsorbent bed height (10–15 cm). High bed height, low flow rate and high initial dye concentration were found to be the better conditions for maximum dye adsorption. To predict the breakthrough curves and to determine the characteristic parameters of the column dynamics for industrial applications and for process design, Thomas model, Yoon–Nelson model and bed depth service time model were applied to experimental breakthrough data. All models were found suitable for describing the dynamic behaviour of the column, with respect to MB flow rate, initial dye concentration and adsorbent bed height. The findings revealed that eucalyptus bark biomass has a high adsorption potential for the removal of MB dye from aqueous solutions in a column system, and that it could be used to treat dye-containing effluents.











Similar content being viewed by others
Abbreviations
- A :
-
Cross sectional area of bed in column (cm2)
- A 1 :
-
Used bed area (cm2)
- A 2 :
-
Unused bed area (cm2)
- a :
-
Slope (N o/C o U)
- b :
-
Intercept (1/K o C o) ln[(C o /C t )−1)]
- C :
-
Effluent MB concentration (mg/L)
- C t :
-
Outlet pollutant concentration (mg/L)
- C o :
-
Inlet pollutant concentration (mg/L)
- H :
-
Height of bed in column (cm)
- H B :
-
Used bed length up to break point (cm)
- H T :
-
Bed height of column (cm)
- H UNB :
-
Unused bed length (cm)
- K o :
-
Rate constant in BDST model (L/mg min)
- K T :
-
Thomas rate constant (mL/mg min)
- K YN :
-
Yoon and Nelson rate constant (min−1)
- MTZ:
-
Mass transfer zone (cm)
- m :
-
Amount of adsorbent in the column (g)
- m p :
-
Mass of pine cone (g)
- m total :
-
Total amount of methylene blue dye sent to column (g)
- N o :
-
Adsorption capacity (mg/L)
- Q :
-
Volumetric flow rate (mL/min)
- q total :
-
Total adsorbed methylene blue dye quantity (g)
- q o :
-
Equilibrium adsorbate uptake (mg/g)
- t :
-
Breakthrough (sampling) time (min)
- t t :
-
Total time (min)
- t total :
-
Total flow time (min)
- t b :
-
Usable capacity of bed up to the breakthrough point time (min)
- t u :
-
Time equivalent to usable capacity (min)
- U :
-
Influent linear velocity (cm/min)
- V :
-
Effluent volume (ml)
- V eff :
-
Total effluent volume (mL)
- τ :
-
Time required for 50 % adsorbate breakthrough (min)
References
E.N. El Qada, S.J. Allen, G.M. Walker, Chem. Eng. J. 135, 174 (2008)
G. Crini, Bioresour. Technol. 97, 1061 (2006)
S.J. Allen, G. Mckay, J.F. Porter, J. Colloid Interface Sci. 280, 322 (2004)
R. Han, Y. Wang, W. Yu, W. Zou, J. Shi, H. Liu, J. Hazard. Mater. 141, 713 (2007)
T. Robinson, G. McMullan, R. Marchant, P. Nigam, Bioresour. Technol. 77, 247 (2001)
R. Gong, Y. Ding, M. Li, C. Yang, H. Liu, Y. Sun, Dyes Pigments 64, 187 (2005)
M.T. Yagub, T.K. Sen, S. Afroze, H. Ang, Adv. Colloid Interface Sci. 209, 172 (2014)
Z. Aksu, Process Biochem. 40, 997 (2005)
G.M. Walker, L.R. Weatherley, Water Res. 31, 2093 (1997)
V. Vadivelan, K.V. Kumar, J. Colloid Interface Sci. 286, 90 (2005)
R. Han, Y. Wang, P. Han, J. Shi, J. Yang, Y. Lu, J. Hazard. Mater. 137, 550 (2006)
V. Garg, M. Amita, R. Kumar, R. Gupta, Dyes Pigments 63, 243 (2004)
T.K. Sen, S. Afroze, H. Ang, Water Air Soil Pollut. 218, 499 (2011)
L. Kong, L. Gong, J. Wang, Desalin. Water Treat. 53, 2489 (2015)
M.T. Yagub, T.K. Sen, H. Ang, Water Air Soil Pollut. 223, 5267 (2012)
L. Zhou, J. Huang, B. He, F. Zhang, H. Li, Carbohydr. Polym. 101, 574 (2014)
Y. Zhao, Y. Xia, H. Yang, Y. Wang, M. Zhao, Desalin. Water Treat. 52, 199 (2014)
M.S. Kini, M. Saidutta, V.R. Murty, Int. J. Chem. Eng. 2014, 1–13 (2014)
G. Akkaya, F. Güzel, Chem. Eng. Commun. 201, 557 (2014)
M. Rafatullah, O. Sulaiman, R. Hashim, A. Ahmad, J. Hazard. Mater. 177, 70 (2010)
T. Chuah, A. Jumasiah, I. Azni, S. Katayon, S. Thomas Choong, Desalination 175, 305 (2005)
S. Afroze, T.K. Sen, M. Ang, H. Nishioka, Desalin. Water Treat. 2015, 1 (2015)
M.T. Yagub, T.K. Sen, S. Afroze, H.M. Ang, Desalin. Water Treat. 2014, 1 (2014)
I. Mall, V. Srivastava, G. Kumar, I. Mishra, Colloids Surf. A Physicochem. Eng. Asp. 278, 175 (2006)
S. Ghorai, K. Pant, Sep. Purif. Technol. 42, 265 (2005)
S. Baral, N. Das, T. Ramulu, S. Sahoo, S. Das, G.R. Chaudhury, J. Hazard. Mater. 161, 1427 (2009)
M.L. Bao, O. Griffini, D. Santianni, K. Barbieri, D. Burrini, F. Pantani, Water Res. 33, 2959 (1999)
S.D. Faust, O.M. Aly, Adsorption Processes for Water Treatment (Elsevier, Amsterdam, 2013)
G. Walker, L. Weatherley, Sep. Sci. Technol. 35, 1329 (2000)
Y. Al-Degs, M. Khraisheh, S. Allen, M. Ahmad, J. Hazard. Mater. 165, 944 (2009)
J. Cruz-Olivares, C. Pérez-Alonso, C. Barrera-Díaz, F. Ureña-Nuñez, M. Chaparro-Mercado, B. Bilyeu, Chem. Eng. J. 228, 21 (2013)
S.T. Ghomshe, S. Mousavi, M. Soltanieh, A.S. Kordi, Sci. Res. Essays 6, 3553 (2011)
Z. Aksu, F. Gönen, Process Biochem. 39, 599 (2004)
J. Goel, K. Kadirvelu, C. Rajagopal, V.K. Garg, J. Hazard. Mater. 125, 211 (2005)
R. Han, J. Zhang, W. Zou, H. Xiao, J. Shi, H. Liu, J. Hazard. Mater. 133, 262 (2006)
R. Han, W. Zou, H. Li, Y. Li, J. Shi, J. Hazard. Mater. 137, 934 (2006)
I. Mobasherpour, E. Salahi, A. Asjodi, Soil Water 4, 5 (2014)
H.C. Thomas, J. Am. Chem. Soc. 66, 1664 (1944)
A. Ahmad, B. Hameed, J. Hazard. Mater. 175, 298 (2010)
C.M. Futalan, C.-C. Kan, M.L. Dalida, C. Pascua, M.-W. Wan, Carbohydr. Polym. 83, 697 (2011)
S. Hasan, D. Ranjan, M. Talat, J. Hazard. Mater. 181, 1134 (2010)
Z. Chowdhury, S. Zain, A. Rashid, R. Rafique, K. Khalid, J. Chem. 2013, 1–8 (2012)
Y.H. Yoon, J.H. Nelson, Am. Ind. Hyg. Assoc. J. 45, 509 (1984)
R. Hutchins, Chem. Eng. 80, 133 (1973)
S. Sadaf, H.N. Bhatti, J. Taiwan Inst. Chem. E 45, 541 (2014)
L.S. Oliveira, A.S. Franca, T.M. Alves, S.D. Rocha, J. Hazard. Mater. 155, 507 (2008)
D.C. Ko, J.F. Porter, G. McKay, Chem. Eng. Sci. 55, 5819 (2000)
C. Djelloul, O. Hamdaoui, Desalin. Water Treat. 2014, 1 (2014)
L. Markovska, V. Meshko, V. Noveski, Korean J. Chem. Eng. 18, 190 (2001)
M.S. Reddy, V. Nirmala, Arab. J. Chem. (2014). doi:10.1016/j.arabjc.2014.08.026
K.S. Bharathi, S.P.T. Ramesh, Appl. Water Sci. 3, 673 (2013)
M. Hadi, M.R. Samarghandi, G. McKay, Water Air Soil Pollut. 218, 197 (2011)
T. Padmesh, K. Vijayaraghavan, G. Sekaran, M. Velan, J. Hazard. Mater. 125, 121 (2005)
S. Chowdhury, R. Mishra, P. Saha, P. Kushwaha, Desalination 265, 159 (2011)
O. Hamdaoui, J. Hazard. Mater. 138, 293 (2006)
P.D. Saha, S. Chowdhury, M. Mondal, K. Sinha, Sep. Sci. Technol. 47, 112 (2012)
S. Sadaf, H.N. Bhatti, Clean Technol. Environ. Policy 16, 527 (2014)
W. Li, Q. Yue, P. Tu, Z. Ma, B. Gao, J. Li, X. Xu, Chem. Eng. J. 178, 197 (2011)
P.D. Saha, S. Chakraborty, S. Chowdhury, Colloids Surf. B Biointerfaces 92, 262 (2012)
I. Tan, A. Ahmad, B. Hameed, Desalination 225, 13 (2008)
D.O. Cooney, Adsorption Design for Wastewater Treatment (CRC Press, Boca Raton, 1998)
Acknowledgments
The Authors would like to thank the Chemical Engineering Department of Curtin University-Perth for financial support, and Chemical Engineering laboratory technicians and Centre of Materials Research (CMR) for their support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Afroze, S., Sen, T.K. & Ang, H.M. Adsorption performance of continuous fixed bed column for the removal of methylene blue (MB) dye using Eucalyptus sheathiana bark biomass. Res Chem Intermed 42, 2343–2364 (2016). https://doi.org/10.1007/s11164-015-2153-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2153-8