Abstract
This paper explains how with the help of nano titania-supported sulfonic acid (n-TSA), new 1,8-dioxo-octahydroxanthene and tetrahydrobenzo[b]pyran derivatives can be produced. To do this, at first n-TSA, which is relatively cheap, easy separable and reusable was made. Next, using this nano catalyst,1,8-dioxo-octahydroxanthene and tetrahydrobenzo[b]pyran derivatives were synthesized from di-ketones and various aromatic aldehydes/or malononitrile without using solvent. Appropriate one-pot operation using different aromatic aldehydes with both electron donating and withdrawing groups have resulted in the best yields. Reusability of the nano catalyst, environmental friendliness, reduced reaction time, non-toxic reaction medium and catalyst high activity are substantial advantages of this work. Nowadays, it is really important that the catalyst can be recovered easily and reused five times while maintaining catalytic activity.












Similar content being viewed by others
Change history
13 January 2024
A Correction to this paper has been published: https://doi.org/10.1007/s11164-023-05209-x
References
Z. Abbasi, S. Rezayati, M. Bagheri, R. Hajinasiri, Chin. Chem. Lett. 28, 75–82 (2017)
S. Madhukar Patil, Curr. Org. Chem. 21(9), 821–833 (2017)
M. Rahimifard, Gh Mohammadi Ziarani, A. Badiei, Sh Asadi, A. Abolhasani Soorki, Res. Chem. Intermed. 42, 3847–3861 (2016)
D. Kumar, V.B. Reddy, Sh Sharad, U. Dube, S. Kapur, Eur. J. Med. Chem. 44, 3805–3809 (2009)
Y.B. Song, Y.H. Yang, J. You, B. Liu, L.J. Wu, Y.L. Hou, W.J. Wang, J.X. Zhu, Chem. Pharm. Bull. 61, 167–175 (2013)
M. Seifi, H. Sheibani, Catal. Lett. 126, 275–279 (2008)
R.W. Lambert, J.A. Martin, J.H. Merrett, K.E.B. Parkes, G.J. Thomas, PCT Int. Appl. WO 9706178. Chem. Abstr. 126, 212377y (1997)
A.G. Banerjee, L.P. Kothapalli, P.A. Sharma, A.B. Thomas, R.K. Nanda, S.K. Shrivastava, V.V. Khatanglekar, Arab. J. Chem. 9, S480–S489 (2016)
J.P. Poupelin, G. Saint-Rut, O. Fussard-Blanpin, G. Narciss, G.U. Ernouf, R. Lakroix, Eur. J. Med. Chem. 13, 67–71 (1978)
H. Qi, B.W. Zhu, N. Abe, Y. Shin, Y. Murata, Y. Nakamura, Food Chem. Toxicol. 50, 1841–1847 (2012)
S. De, A. Das, A. Girigoswami, Spectrochim. Acta Part A 61, 1821–1833 (2005)
C.C. Chang, Y.T. Yang, H.D. Wu, T. Tsai, Dyes Pigm. 79, 170–175 (2008)
B. Karami, Z. Zare, K. Eskandari, Chem. Pap. 67, 145–154 (2013)
H.S. Haeri, S. Rezayati, E. Rezaee Bezhad, H. Darvishi, Res. Chem. Intermed. 42, 4773–4784 (2016)
S. Rezayati, Z. Erfani, R. Hajinasiri, Chem. Pap. 69, 536–543 (2015)
F. Shirini, M. Abedini, R. Pourhasan, Dyes Pigm. 99(1), 250–255 (2013)
Z. Zhou, X. Deng, J. Mol. Catal. A Chem. 367, 99–102 (2013)
A. Pramanik, S. Bhar, Catal. Commun. 20, 17–24 (2012)
K. Niknam, M. Damya, J. Chin. Chem. Soc. 56(3), 659–665 (2009)
H.R. Shaterian, A. Hosseinian, M. Ghashang, Turk. J. Chem. 33(2), 233–240 (2009)
K. Vankatesan, S.S. Pujari, R.J. Lahoti, K.V. Srini-vasan, Ultrason. Sonochem. 15(4), 548–553 (2008)
S. Makone, Sh Mahurkar, Green Sustain. Chem. 3, 27–32 (2013)
S. Selva Ganesan, J. Kothandapani, A. Ganesan, Lett. Org. Chem. 11, 682–687 (2016)
S.S. Pourpanah, S.M. Habibi-Khorassani, M. Shaharaki, Chin. J. Catal. 36, 757–763 (2015)
J. Albadi, A. Mansournezhad, Z. Derakhshandeh, Chin. Chem. Lett. 24, 821–824 (2013)
S. Nemouchi, R. Boulcina, B. Carboni, A. Debache, C. R. Chim. 15, 394–397 (2012)
J. Zheng, Y. Li, Arch. Appl. Sci. Res. 3(2), 381–388 (2011)
N. Koukabi, E. Kolvari, A. Khazaei, M.A. Zolfigol, B. Shirmardi-Shaghasemi, H.R. Khavas, Chem. Commun. 47, 9230–9232 (2011)
N. Koukabi, E. Kolvari, M.A. Zolfigol, A. Khazaei, B.S. Shaghasemi, B. Fasahati, Adv. Synth. Catal. 354, 2001–2008 (2012)
S. Rahmani, A. Amoozadeh, E. Kolvari, Catal. Commun. 56, 184–188 (2014)
A. Amoozadeh, E. Tabrizian, S. Rahmani, C. R. Chim. 18, 848–857 (2015)
A. Amoozadeh, S. Rahmani, M. Hafezi, E. Tabrizian, E. Imanifar, F. Zolfagharkhani, React. Kinet. Mech. Catal. 117, 365–367 (2016)
N. Venkatachalam, M. Palanichamy, V. Murugesan, J. Mol. Catal. A Chem. 273, 177–185 (2007)
H.R. Shaterian, M. Ghashang, M. Feyzi, Appl. Catal. A Gen. 345, 128–133 (2008)
H. Xing, T. Wang, Z. Zhou, Y. Dai, J. Mol. Catal. A Chem. 264, 53–59 (2007)
S. Khaksar, A. Rouhollahpour, S. Mohammadzadeh Talesh, J. Fluor. Chem. 141, 11–15 (2012)
Gh Mohammadi Ziarani, A. Abbasi, A. Badiei, Z. Aslani, Eur. J. Chem. 8(1), 293–299 (2011)
S. Banerjee, A. Horn, H. Khatri, G. Sereda, Tetrahedron Lett. 52, 1878–1881 (2011)
J.-C. Xu, W.-M. Li, H. Zheng, Y.-F. Lai, P.-F. Zhang, Tetrahedron 67, 9582–9587 (2011)
I. Devi, P.J. Bhuyan, Tetrahedron Lett. 45, 8625–8627 (2004)
S. Kantevari, R. Bantu, L. Nagarapu, J. Mol. Catal. A Chem. 269, 53–57 (2007)
T.S. Jin, J.S. Zhang, A.Q. Wang, T.S. Li, Ultrason. Sonochem. 13, 220–224 (2006)
S. Rostamizadeh, A.M. Amani, G.H. Mahdavinia, G. Amiri, H. Sepehrian, Ultrason. Sonochem. 17, 306–309 (2010)
S. Gowravaram, K. Arundhathi, K. Sudhakar, B.S. Sastry, J.S. Yadav, Synth. Commun. 38, 3439–3446 (2008)
A. Javid, M.M. Heravi, F.F. Bamoharram, Eur. J. Chem. 8(2), 910–916 (2011)
R. Khoeiniha, A. Ezabadi, A. Olyaei, Iran. Chem. Commun. 4, 273–282 (2016)
S.N. Sadat, F. Hatam, Jafari. Orient. J. Chem. 31(2), 1191–1193 (2015)
S.S. Kahandal, A.S. Burange, S.R. Kale, P. Prinsen, R. Luque, R.V. Jayaram, Catal. Commun. 97, 138–145 (2017)
J. Zheng, Y. Li, Mendeleev Commun. 21, 280–281 (2011)
T. Jin, A. Wang, F. Shi, L. Han, L. Liu, T. Li, ARKIVOC 14(xiv), 78–86 (2006)
R. Hekmatshoar, S. Majedi, K. Bakhtiari, Catal. Commun. 9, 307–310 (2008)
S. Balalaie, M. Sheikh-Ahmadi, M. Bararjanian, Catal. Commun. 8, 1724–1728 (2007)
B. Boumoud, A.A. Yahiaoui, T. Boumoud, A. Debache, J. Chem. Pharm. Res. 4(1), 795–799 (2012)
A.A. Mohammadi, M.R. Asghariganjeh, A. Hadadzahmatkesh, Arab. J. Chem. 10, S2213–S2216 (2017)
L. Wang, J. Shao, H. Tian, Y. Wang, B. Liu, J. Fluor. Chem. 127, 97–100 (2006)
E. Sheikhhosseini, D. Ghazanfari, V. Nezamabadi, Iran. J. Catal. 3(4), 197–201 (2013)
Acknowledgements
We thank the Department of Chemistry of Semnan University for supporting this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
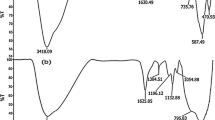
The original online version of this article was revised: The Fig. 7 has been corrected.
Rights and permissions
About this article
Cite this article
Amoozadeh, A., Hosseininya, S. & Rahmani, S. Nano titania-supported sulfonic acid (n-TSA) as an efficient, inexpensive, and reusable catalyst for one-pot synthesis of 1, 8-dioxo-octahydroxanthene and tetrahydrobenzo[b]pyran derivatives. Res Chem Intermed 44, 991–1011 (2018). https://doi.org/10.1007/s11164-017-3148-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-3148-4