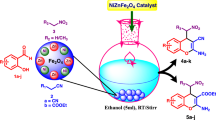
Abstract
In this study, firstly, CuFe2O4 nanoparticles were prepared by a simple operation. The structure of the mentioned nanoparticles was characterized by Fourier transform infrared spectroscopy, X-ray diffraction, scanning electron microscopy, transmission electron microscopy, energy-dispersive X-ray spectroscopy, inductively coupled plasma-optical emission spectrometry, vibrating sample magnetometer and also Brunauer–Emmett–Teller and Barrett–Joyner–Halenda analyses. The prepared magnetically copper ferrite nanocomposite was successfully applied as a simple, cost-effective, practicable, and recoverable catalyst on the green, highly efficient, fast, base-free, and ligand-free reduction of nitriles and also on the affordable and eco-friendly reduction of nitro compounds with the broad substrate scope to the corresponding amines with NaBH4 in water at reflux in high to excellent yields.
Graphical abstract



















Similar content being viewed by others
References
T. Senthamarai, K. Murugesan, K. Natte, N.V. Kalevaru, H. Neumann, P.C.J. Kamer, R.V. Jagadeesh, ChemCatChem 10, 1235 (2018)
I.B. Seiple, S. Su, I.S. Young, C.A. Lewis, J. Yamaguchi, P.S. Baran, Angew. Chem. Int. Ed. 49, 1095 (2010)
A. Gomm, E. O’Reilly, Curr. Opin. Chem. Biol. 43, 106 (2018)
R.S. Kokane, V.R. Acham, A.B. Kulal, E. Kemnitz, M.K. Dongare, S.B. Umbarkar, ChemistrySelect 2, 10618 (2017)
M. Rimaz, H. Mousavi, M. Behnam, B. Khalili, Curr. Chem. Lett. 5, 145 (2016)
M. Rimaz, B. Khalili, G. Khatyal, H. Mousavi, F. Aali, Aust. J. Chem. 70, 1274 (2017)
R. Madhavachary, T. Zarganes-Tłuścik, A. Dömling, ACS Comb. Sci. 20, 192 (2018)
R. Bansal, P.K. Soni, A.K. Halve, J. Heterocycl. Chem. 55, 1308 (2018)
Y. Selim, M.H.M.A. El-Azim, J. Heterocycl. Chem. 55, 1403 (2018)
R. Saini, S.R. Malladi, N. Dharavath, J. Heterocycl. Chem. 55, 1579 (2018)
A. Ghorbani-Choghamarani, Z. Taherinia, Aust. J. Chem. 70, 1127 (2017)
H. Kandemir, I.F. Sengul, N. Kumar, D.S. Black, Aust. J. Chem. 70, 1196 (2017)
G. Khanna, P. Saluja, J.M. Khurana, Aust. J. Chem. 70, 1285 (2017)
B. Xu, J. Su, J. Wang, G.-C. Zhou, Aust. J. Chem. 69, 770 (2016)
Y. Kuninobu, Y. Nishina, M. Shouho, K. Takai, Angew. Chem. 118, 2832 (2006)
M. Rimaz, H. Mousavi, M. Behnam, L. Sarvari, B. Khalili, Curr. Chem. Lett. 6, 55 (2017)
A. Saeed, P.A. Channar, J. Heterocycl. Chem. 54, 780 (2017)
M. Rimaz, H. Mousavi, L. Nikpey, B. Khalili, Res. Chem. Intermed. 43, 3925 (2017)
S.H. Mccooey, S.J. Connon, Org. Lett. 9, 599 (2007)
J. Ou-Yang, Y. Zhao, H. Jiang, L. Meng, X. Li, X. Jia, Aust. J. Chem. 68, 1599 (2015)
M. Rimaz, H. Mousavi, Turk. J. Chem. 37, 252 (2013)
A. Komaromi, G.L. Tolnai, Z. Novak, Tetrahedron Lett. 49, 7294 (2008)
S. Uppuluri, S.E. Keinath, D.A. Tomalia, P.R. Dvornic, Macromolecules 31, 4498 (1998)
L.H. Amundsen, L.S. Nelson, J. Am. Chem. Soc. 73, 242 (1951)
T. Satoh, S. Suzuki, Y. Suzuki, Y. Miyaji, Z. Imai, Tetrahedron Lett. 10, 4555 (1969)
S.W. Heinzman, B. Ganem, J. Am. Chem. Soc. 104, 6801 (1982)
J.O. Osby, S.W. Heinzman, B. Ganem, J. Am. Chem. Soc. 108, 67 (1986)
J.M. Khurana, G. Kukreja, Synth. Commun. 32, 1265 (2002)
J.Z. Saavedra, A. Resendez, A. Rovira, S. Eagon, D. Haddenham, B. Singaram, J. Org. Chem. 77, 221 (2012)
A.S. Bhanu Prasad, J.V. Bhaskar Kanth, M. Periasamy, Tetrahedron 48, 4623 (1992)
D. Haddenham, L. Pasumansky, J. DeSoto, S. Eagon, B. Singaram, J. Org. Chem. 74, 1646 (2009)
S. Thomas, C.J. Collins, J.R. Cuzens, D. Spieciarich, C.T. Goralski, B. Singaram, J. Org. Chem. 66, 1999 (2001)
B. Wu, J. Zhang, M. Yang, Y. Yue, L.-J. Ma, X.-Q. Yu, Arkivoc xii, 95 (2008)
H.C. Brown, Y.M. Choi, S. Narasimhan, Synthesis 605 (1981)
A. Nose, T. Kudo, Chem. Pharm. Bull. 38, 1720 (1990)
N. Gandhamsetty, J. Jeong, J. Park, S. Park, S. Chang, J. Org. Chem. 80, 7281 (2015)
I. Cabrita, A.C. Fernandes, Tetrahedron 67, 8183 (2011)
C. Bornschein, S. Werkmeister, K. Jungea, M. Beller, New J. Chem. 37, 2061 (2013)
S. Laval, W. Dayoub, A. Favre-Reguillon, M. Berthod, P. Demonchaux, G. Mignani, M. Lemaire, Tetrahedron Lett. 50, 7005 (2009)
X. Chen, S. Zhou, C. Qian, Arkivoc viii, 128 (2012)
M. Szostak, B. Sautier, M. Spain, D.J. Procter, Org. Lett. 16, 1092 (2014)
A. Muthukumaran, V. Krishnan, Bull. Electrochem. 7, 410 (1991)
W.H. Carothers, G.A. Jones, J. Am. Chem. Soc. 47, 3051 (1925)
F.E. Gould, G.S. Johnson, A.F. Ferris, J. Org. Chem. 25, 1658 (1960)
M. Freifelder, J. Am. Chem. Soc. 82, 2386 (1960)
S. Takamizawa, N. Wakasa, T. Fuchikami, Synlett 10, 1623 (2001)
S. Enthaler, K. Junge, D. Addis, G. Erre, M. Beller, ChemSusChem 1, 1006 (2008)
S. Das, S. Zhou, D. Addis, S. Enthaler, K. Junge, M. Beller, Top. Catal. 53, 979 (2010)
S. Enthaler, D. Addis, K. Junge, G. Erre, M. Beller, Chem. Eur. J. 14, 9491 (2008)
C. Bornschein, S. Werkmeister, B. Wendt, H. Jiao, E. Alberico, W. Baumann, H. Junge, K. Junge, M. Beller, Nat. Commun. 5, 4111 (2014)
R. Adam, C.B. Bheeter, R. Jackstell, M. Beller, ChemCatChem 8, 1329 (2016)
X. Miao, J. Bidange, P.H. Dixneuf, C. Fischmeister, C. Bruneau, J.-L. Dubois, J.-L. Couturier, ChemCatChem 4, 1911 (2012)
S.J. Tabatabaei Rezaei, A. Mashhadi Malekzadeh, S. Poulaei, A. Ramazani, H. Khorramabadi, Appl. Organomet. Chem. 32, e3975 (2018)
S.J. Tabatabaei Rezaei, H. Khorramabadi, A. Hesami, A. Ramazani, V. Amani, R. Ahmadi, Ind. Eng. Chem. Res. 56, 12256 (2017)
S. Liu, Y. Yang, X. Zhen, J. Li, H. He, J. Feng, A. Whiting, Org. Biomol. Chem. 10, 663 (2012)
K. Tokmic, B.J. Jackson, A. Salazar, T.J. Woods, A.R. Fout, J. Am. Chem. Soc. 139, 13554 (2017)
C. Yu, J. Fu, M. Muzzio, T. Shen, D. Su, J. Zhu, S. Sun, Chem. Mater. 29, 1413 (2017)
D. Addis, S. Enthaler, K. Junge, B. Wendt, M. Beller, Tetrahedron Lett. 50, 3654 (2009)
M.R. Nabid, Y. Bide, M. Niknezhad, ChemCatChem 6, 538 (2014)
R. Ferraccioli, D. Borovika, A.-E. Surkus, C. Kreyenschlte, C. Topf, M. Beller, Catal. Sci. Technol. 8, 499 (2018)
M. Yoshimura, A. Komatsu, M. Niimura, Y. Takagi, T. Takahashi, S. Ueda, T. Ichikawa, Y. Kobayashi, H. Okami, T. Hattori, Y. Sawama, Y. Monguchin, H. Sajik, Adv. Synth. Catal. 360, 1726 (2018)
T. Takao, S. Horikoshi, T. Kawashima, S. Asano, Y. Takahashi, A. Sawano, H. Suzuki, Organometallics 37, 1598 (2018)
A.N. Ajjou, A. Robichaud, Appl. Organomet. Chem. 32, e4481 (2018)
S. Das, B. Wendt, K. Möller, K. Junge, M. Beller, Angew. Chem. 124, 1694 (2012)
A.N. Ajjou, A. Robichaud, Appl. Organomet. Chem. 32, e4547 (2018)
A. Zamani, A. Poursattar Marjani, A. Nikoo, M. Heidarpour, A. Dehghan, Inorg. Nano-Metal Chem. 8, 1 (2019)
M. Sutter, L. Pehlivan, R. Lafon, W. Dayoub, Y. Raoul, E. Métay, M. Lemaire, Green Chem. 15, 3020 (2013)
D. Mullangi, D. Chakraborty, A. Pradeep, V. Koshti, C.P. Vinod, S. Panja, S. Nair, R. Vaidhyanathan, Small 14, 1801233 (2018)
L. Liu, Y. Liu, Y. Ai, J. Li, J. Zhou, Z. Fan, H. Bao, R. Jiang, Z. Hu, J. Wang, K. Jing, Y. Wang, Q. Liang, H. Sun, Science 8, 61 (2018)
H. Göksu, S.F. Ho, Ö. Metin, K. Korkmaz, A.M. Garcia, M.S. Gültekin, S. Sun, ACS Catal. 4, 1777 (2018)
L. Liu, J. Li, Y. Ai, Y. Liu, J. Xiong, H. Wang, Y. Qiao, W. Liu, S. Tan, S. Feng, K. Wang, H. Sun, Q. Liang, Green Chem. (2019). https://doi.org/10.1039/C8GC03595D
Y. Liu, S. He, Z. Quan, H. Cai, Y. Zhao, B. Wang, Green Chem. 21, 830 (2019)
Y. Zhang, H. Yang, Q. Chi, Z. Zhang, ChemSusChem (2019). https://doi.org/10.1002/cssc.201802459
N. Futtahi, M. Ayoubi, A. Ramazani, Tetrahedron 74, 4351 (2018)
H.Y. Lü, S.-H. Yang, J. Deng, Z.-H. Zhang, Aust. J. Chem. 63, 1290 (2010)
M. Rimaz, J. Khalafy, P. Najafi-Moghaddam, Aust. J. Chem. 63, 1396 (2010)
M. Rimaz, J. Khalafy, H. Mousavi, S. Bohlooli, B. Khalili, J. Heterocycl. Chem. 54, 3174 (2017)
M. Rimaz, J. Khalafy, H. Mousavi, Res. Chem. Intermed. 42, 8185 (2016)
M. Rimaz, H. Mousavi, B. Khalili, F. Aali, J. Chin. Chem. Soc. 65, 1389 (2018)
L.M. Rossi, N.J.S. Costa, F.P. Silva, R. Wojcieszak, Green Chem. 16, 2933 (2014)
D. Wang, D. Astruc, Chem. Soc. Rev. 46, 816 (2017)
A.F. Costa, P.M. Pimentel, F.M. Aquino, D.M.A. Melo, M.A.F. Melo, I.M.G. Santos, Mater. Lett. 112, 58 (2013)
Z. Sun, L. Liu, D.Z. Jia, W. Pan, Sens. Actuators B Chem. 125, 144 (2007)
E.R. Kumar, S.P. Reddy, G.S. Devi, S. Sathiyaraj, J. Magn. Magn. Mat. 398, 281 (2016)
H. Mousavi, B. Zeynizadeh, R. Younesi, M. Esmati, Aust. J. Chem. 71, 595 (2018)
B. Zeynizadeh, R. Younesi, H. Mousavi, Res. Chem. Intermed. 44, 7331 (2018)
B. Zeynizadeh, F. Sepehraddin, J. Organomet. Chem. 856, 70 (2018)
B. Zeynizadeh, H. Mousavi, S. Zarrin, J. Chin. Chem. Soc. (2019). https://doi.org/10.1002/jccs.201800325
Acknowledgements
We are thankful to the research council of Urmia University for the partial support of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zeynizadeh, B., Mohammad Aminzadeh, F. & Mousavi, H. Green and convenient protocols for the efficient reduction of nitriles and nitro compounds to corresponding amines with NaBH4 in water catalyzed by magnetically retrievable CuFe2O4 nanoparticles. Res Chem Intermed 45, 3329–3357 (2019). https://doi.org/10.1007/s11164-019-03794-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-019-03794-4