Abstract
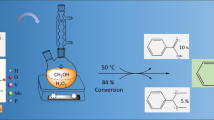
The reactivity of Zn2+ and VO2+ ions towards pyridinyl Schiff bases, in the absence or presence of a p-sodium sulfonate group (HPSNa and HPS, respectively), provided polar and less polar catalyst complexes, which were characterized by various measurements. Stability constants of Zn-complexes [Zn(PS)2 and Zn(PSNa)2], and VO-complexes [VO(PS)2 and VO(PSNa)2] were measured spectrophotometrically. A typical (ep)oxidation processes of 1,2-cyclohexene or cyclohexane using 30% aqueous H2O2 catalyzed by our synthesized catalyst system, were tested. VO-complexes were found to be more effective catalysts than Zn-chelates. The polarity of Zn- and VO-complexes, i.e. the presence of p-SO3Na, displayed an observable influence on their catalytic performance chemoselectively. The polar catalyst system, Zn(PSNa)2 and VO(PSNa)2, in polar solvents such as H2O, MeOH, acetonitrile and acetone exhibited higher catalytic activity towards the (ep)oxidation processes than the less polar catalysts, Zn(PS)2 and VO(PS)2. In a less polar solvent such as CHCl3, polar catalysts showed higher conversion, but low chemoselectively, whereas the less polar catalysts showed relatively higher conversion and chemoselectivity. Under solvent-free conditions, less polar complexes were found to be more efficient catalysts than the polar chelates.








Similar content being viewed by others
References
K.A.D.F. Castro, S. Silva, P.M.R. Pereira, M.M.Q. Simões, M. da Graça, P.M.S. Neves, J.A.S. Cavaleiro, F. Wypych, J.P.C. Tomé, S. Nakagaki, Inorg. Chem. 54, 4382 (2015)
M. Hajrezaie, M. Paydar, C.Y. Looi, S.Z. Moghadamtousi, P. Hassandarvish, M.S. Salga, H. Karimian, K. Shams, M. Zahedifard, N.A. Majid, H.M. Ali, M.A. Abdulla, Sci. Rep. 5, 9097 (2015)
M.S.S. Adam, H. Elsawy, J. Photochem. Photobio. B 184, 34 (2018)
S.-J. Peng, L.-B. Song, J.-H. Ning, Z.-L. Xiao, Synth. React. Inorg. Met.-Org. Chem. 40, 105 (2010)
W. Al Zoubi, N. Al Mohanna, Spectrochim Acta A 132, 854 (2014)
A.W. Jeevadason, K.K. Murugavel, M.A. Neelakantan, Renew. Sustain. Energy Rev. 36, 220 (2014)
W. Al Zoubi, Y.G. Ko, J. Organomet. Chem. 822, 173 (2016)
M. Khorshidifard, H.A. Rudbari, B. Askari, M. Sahihi, M.R. Farsani, F. Jalilian, G. Bruno, Polyhedron 95, 1 (2015)
L.M.D.R.S. Martins, A.J.L. Pombeiro, Eur. J. Inorg. Chem. 15–16, 2236 (2016)
X. Liu, C. Manzur, N. Novoa, S. Celedón, D. Carrillo, J.-R. Hamon, Coord. Chem. Rev. 357, 144 (2018)
M. Sutradhar, L.M.D.R.S. Martins, M.F.C.G. da Silva, A.J.L. Pombeiro, Coord. Chem. Rev. 301–302, 200 (2015)
T.F.S. Silva, K.V. Luzyanin, M.V. Kirillova, M.F.G. da Silva, L.M.D.R.S. Martins, A.J.L. Pombeiro, Adv. Synth. Catal. 352, 171 (2010)
Y. Zhang, L. Zhao, H. Zhang, R. Huang, J. Zhao, Appl. Organomet. Chem. 31, e3709 (2017)
L.M.D.R.S. Martins, A.J.L. Pombeiro, Coord. Chem. Rev. 265, 74 (2014)
J.A.L. da Silva, J.J.R.F. da Silva, A.J.L. Pombeiro, Coord. Chem. Rev. 255, 2232 (2011)
M. Maurya, C. Haldar, A. Kumar, M.L. Kuznetsov, F. Avecilla, J.C. Pessoa, Dalton Trans. 42, 11941 (2013)
J. Adhikary, A. Datta, S. Dasgupta, A. Chakraborty, M.I. Menéndez, T. Chattopadhyay, RSC Adv. 5, 92634 (2015)
S. Rayati, A. Ghaemi, N. Sadeghzadeh, Catal. Commun. 11, 792 (2010)
M. Mandal, V. Nagaraju, G.V. Karunakar, B. Sarma, B.J. Borah, K.K. Bania, J. Phys. Chem. C 119, 28854 (2015)
M. Vlasiou, C. Drouza, T.A. Kabanos, A.D. Keramidas, J. Inorg. Biochem. 147, 39 (2015)
M.S.S. Adam, Appl. Organometal. Chem. 32, e4234 (2018)
T.F.S. Silva, B.G.M. Rocha, M.F.G. da Silva, L.M.D.R.S. Martins, A.J.L. Pombeiro, New J. Chem. 40, 528 (2016)
M. Sutradhar, A.J.L. Pombeiro, Coord. Chem. Rev. 265, 89 (2014)
G. Licini, V. Conte, A. Coletti, M. Mba, C. Zonta, Coord. Chem. Rev. 255, 2345 (2011)
O. Bortolini, V. Conte, Mass Spectrom. Rev. 25, 724 (2006)
L. Gómez, I. Garcia-Bosch, A. Company, J. Benet-Buchholz, A. Polo, X. Sala, X. Ribas, M. Costas, Angew. Chem. Int. Ed. 48, 5720 (2009)
M.R.M. Bisht, A. Kumar, M.L. Kuznetsov, F. Avecilla, J.C. Pessoa, Dalton Trans. 40, 6968 (2011)
M. Sedighipoor, A.H. Kianfar, W.A.K. Mahmood, M.H. Azarian, Inorg. Chim. Acta 457, 116 (2017)
S. Enthaler, X.-F. Wu, M. Weidauer, E. Irran, P. Döhlert, Inorg. Chem. Commun. 46, 320 (2014)
R. Borthakur, M. Asthana, A. Kumar, R.A. Lal, Inorg. Chem. Commun. 46, 198 (2014)
S. Bertini, A. Colette, B.Y. Floris, V. Conte, P. Galliani, J. Inorg. Biochem. 147, 44 (2015)
J. Adhikary, A. Datta, S. Dasgupta, A. Chakraborty, M.I. Menendez, T. Chattopadhyay, RSC Adv. 5, 92634 (2015)
G. Romanowski, J. Kira, M. Wera, Polyhedron 67, 529 (2014)
G. Grivani, V. Tahmasebi, A.D. Khalaji, K. Fejfarová, M. Dušek, Polyhedron 51, 54 (2013)
M.S.S. Adam, A.D.M. Mohamad, Polyhedron 151, 118 (2018)
R.H. Crabtree, J. Organomet. Chem. 689, 4083 (2004)
G.B. Shul’pin, J. Mol. Catal. A 189, 39 (2002)
A.A. Alshaheri, M.I.M. Tahir, M.B. Abdul Rahman, T.B.S.A. Ravoof, T.A. Saleh, Chem. Eng. J. 327, 423 (2017)
A.A. Alshaheri, M.I.M. Tahir, M.B. Abdul Rahman, T. Begum, T.A. Saleh, J. Mol. Liq. 240, 486 (2017)
H.M. Abd El-Lateef, M.S.S. Adam, M.M. Khalaf, J. Taiwan Inst. Chem. Eng. 88, 286 (2018)
C.D. Nunes, P.V. Vaz, V. Felix, L.F. Veiros, T. Moniz, M. Rangel, S. Realista, A.C. Mourato, M.J. Calhorda, Dalton Trans. 44, 5125 (2015)
M.S.S. Adam, H.M. Abd El-Lateef, K.A. Soliman, J. Mol. Liq. 250, 207 (2018)
M. Enamullah, M.A. Quddus, M.M. Rahman, T.E. Burrow, J. Mol. Struct. 1130, 765 (2017)
M.T. Beck, I. Nagypál, Chemistry of Complex Equilibria (Akadémiai Kiadó, Budapest, 1990)
G. Lante, Curr. Opin. Chem. Eng. 21, 76 (2018)
G. Marinescu, A.M. Madalan, M. Andruh, J. Coord. Chem. 68, 479 (2015)
C. Alvariño, J. Romero, A. Sousa, M.L. Durán, ZAAC 556, 223 (1988)
G.A. Kolawole, J. Coord. Chem. 16, 67 (1987)
M.S.S. Adam, A.M. Hafez, I. El-Ghamry, Reac. Kinet. Mech. Cat. 124, 779 (2018)
M.S.S. Adam, Monatsh. Chem. 146, 1823 (2015)
P. Adão, J.C. Pessoa, R.T. Enriques, M.L. Kuznetsov, F. Avecilla, M.R. Maurya, U. Kumar, I. Correia, Inorg. Chem. 48, 3542 (2009)
A. Di Giuseppe, C. Di Nicola, R. Pettinari, I. Ferino, D. Meloni, M. Passacantando, M. Crucianelli, Catal. Sci. Technol. 3, 1972 (2013)
H.H. Monfared, R. Bikas, P. Mayer, Inorg. Chim. Acta 363, 2574 (2010)
C. Cordelle, D. Augustin, J.-C. Daran, R. Poli, Inorg. Chim. Acta 364, 144 (2010)
S.A. Talouki, G. Grivani, A.D. Khalaji, Appl. Organomet. Chem. 31, e4078 (2017)
J. Rahchamani, M. Behzad, A. Bezaatpour, V. Jahed, G. Dutkiewicz, M. Kubicki, M. Salehi, Polyhedron 30, 2611 (2011)
S. Rayati, F. Ashouri, C. R. Chim. 15, 679 (2012)
A. Coletti, P. Galloni, A. Sartorel, V. Conte, B. Floris, Catal. Today 192, 44 (2012)
P. Galloni, A. Coletti, B. Floris, V. Conte, Inorg. Chim. Acta 420, 144 (2014)
K. Pamin, G. Pozzi, E. Tabor, W. Bukowski, J. Połtowicz, Catal. Commun. 39, 102 (2013)
M. Saha, K.M. Vyas, L.M.D.R.S. Martins, N.M.R. Martins, A.J.L. Pombeiro, S.M. Mobin, D. Bhattacherjee, K.P. Bhabak, S. Mukhopadhyay, Polyhedron 132, 53 (2017)
G.B. Shul’pin, Dalton Trans. 42, 12794 (2013)
A.R. Silva, T. Mourão, J. Rocha, Catal. Today 203, 81 (2013)
V. Conte, A. Coletti, B. Floris, G. Licini, C. Zonta, Coord. Chem. Rev. 255, 2165 (2011)
L.P. de Carvalho, K.F. Silva, L.L. dos Santos, M.V.P. dos Santos, J.A.B. da Silva, R.L. Longo, Inorg. Chim. Acta 467, 351 (2017)
Acknowledgements
The authors greatly thank the Vice-President of Graduate Studies and Academic Research in King Faisal University for their financial support and encouragement to produce this work as a scientific project (Project No. 17122005).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Adam, M.S.S., Al-Omair, M.A. & Ullah, F. Catalytic comparison of various polar Zn(II)-Schiff base complexes and VO(II)-Schiff base complexes in (ep)oxidation processes of 1,2-cyclohexene and cyclohexane. Res Chem Intermed 45, 4653–4675 (2019). https://doi.org/10.1007/s11164-019-03855-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-019-03855-8