Abstract
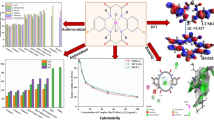
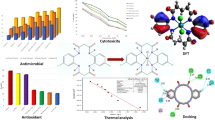
A series of N-dibenzosuberene substituted aroyl selenourea ligands L1–L3 and their Ru(II) (η6-p-cymene) complexes 1–3, [Ru(II) (η6-p-cymene) L] (L = monodentate aroyl selenourea ligand) were synthesized and characterized. The molecular structures of the ligand L3 and complex 3 were confirmed by single-crystal XRD method. The single-crystal XRD study results revealed that aroyl selenourea ligand coordinates with ruthenium via Se neutral monodentate atom. In vitro DNA interaction studies were investigated by UV–Visible and fluorescence spectroscopic methods which showed that the intercalative mode of binding is in the order of 3 > 2 > 1 with the Ru(II) (η6-p-cymene) complexes. The binding affinity of the bovine serum albumin with complexes was calculated using spectroscopic methods. Quantum chemical computations were made using DFT (density functional theory), BL3YP; LANL2DZ basis set in order to determine the frontier molecular orbital parameters and MESP for the newly synthesized complexes. The complexes 1–3 have shown intensive cytotoxicity against the cancer lines HepG-2 and A549 under in vitro conditions. Complex 3 (IC50 = 62 μM) has shown significant cytotoxic activity against HepG-2 compared to cisplatin standard drug. The complexes also examined for their antimicrobial activity. The complex 2 exhibited good activity against B. subtilis (MIC: 13.60 μg/mL), E. coli (MIC: 8.01 μg/mL) and A. flavus (MIC = 15.60 μg/mL), respectively, compared to reference drugs Streptomycin and Ketoconazole.














Similar content being viewed by others
References
M. Ohmichi, J. Hayakawa, K. Tasaka, H. Kurachi, Y. Murata, Trends Pharmacol. Sci. 26, 113 (2005)
M.A. Fernández-Herrera, J. Sandoval-Ramírez, L. Sánchez-Sánchez, H. López-Muñoz, M.L. Escobar-Sánchez, Eur. J. Med. Chem. 74, 451 (2014)
W. Guo, W. Zheng, Q. Luo, X. Li, Y. Zhao, S. Xiong, F. Wang, Inorg. Chem. 52, 5328 (2013)
C. Gossens, I. Tavernelli, U. Rothlisberger, J. Am. Chem. Soc. 130, 10921 (2008)
A.C. Komor, J.K. Barton, Chem. Commun. 49, 3617 (2013)
N.P.E. Barry, P.J. Sadler, Chem. Commun. 49, 5106 (2013)
A.F.A. Peacock, P.J. Sadler, Chem. Asian J. 3, 1890 (2008)
G.S. Smith, B. Therrien, Dalton Trans. 40, 10793 (2011)
M.J. Clarke, Coord. Chem. Rev. 236, 209 (2003)
G. Sava, G. Jaouen, E.A. Hillard, A. Bergamo, Dalton Trans. 41, 8226 (2012)
C.A. Vock, A.K. Renfrew, R. Scopelliti, L. Juillerat-Jeanneret, P.J. Dyson, Eur. J. Inorg. Chem. 2008, 1661 (2008)
A. Dorcier, C.G. Hartinger, R. Scopelliti, R.H. Fish, B.K. Keppler, P.J. Dyson, J. Inorg. Biochem. 102, 1066 (2008)
K.J. Kilpin, S. Crot, T. Riedel, J.A. Kitchen, P.J. Dyson, Dalton Trans. 43, 1443 (2014)
J. Bravo, S. Bolaño, L. Gonsalvi, M. Peruzzini, Coord. Chem. Rev. 254, 555 (2010)
C.A. Vock, C. Scolaro, A.D. Phillips, R. Scopelliti, G. Sava, P.J. Dyson, J. Med. Chem. 49, 5552 (2006)
K. Jeyalakshmi, J. Haribabu, N.S.P. Bhuvanesh, R. Karvembu, Dalton Trans. 45, 12518 (2016)
H. Mertz, J. Clin. Gastroenterol. 39, 247 (2005)
A. Hjelmencrantz, A. Friberg, U. Berg, J. Chem. Soc. Perkin Trans. 2, 1293 (2000)
C. Tuchila, D. Baconi, C. Pirvu, D. Balalau, A. Vlasceanu, M. Stan, C. Balalau, J. Mind Med. Sci. 4, 100 (2017)
J.C. Burbiel, Arkivoc 2006, 16 (2006)
S. Merkaš, M. Litvić, I. Cepanec, V. Vinković, Molecules 10, 1429 (2005)
S.C. Koeberle, S. Fischer, D. Schollmeyer, V. Schattel, C. Grütter, D. Rauh, S.A. Laufer, J. Med. Chem. 55, 5868 (2012)
G. Rohini, K. Ramaiah, K.N. Aneesrahman, M.C. Aryasenan, N.S.P. Bhuvanesh, K.L. Reddy, A. Sreekanth, Appl. Organomet. Chem. 32, e4567 (2018)
G. Rohini, J. Haribabu, M.M. Sheeba, K.N. Aneesrahman, N.S.P. Bhuvanesh, C. Balachandran, R. Karvembu, A. Sreekanth, ChemistrySelect 3, 18 (2018)
G. Rohini, J. Haribabu, K.N. Aneesrahman, N.S.P. Bhuvanesh, K. Ramaiah, R. Karvembu, A. Sreekanth, Polyhedron 152, 147 (2018)
S. Swaminathan, J. Haribabu, N.K. Kalagatur, R. Konakanchi, N. Balakrishnan, N. Bhuvanesh, R. Karvembu, ACS Omega 4, 6245 (2019)
G.N. Babu, A.R.B. Rao, S. Keesara, S. Pal, J. Organomet. Chem. 848, 243 (2017)
S.J. Pradeepa, N. Sundaraganesan, Spectrochim. Acta A 125, 211 (2014)
M. Ramesh, G. Venkatachalam, J. Organomet. Chem. 880, 47 (2019)
M. Patra, T. Joshi, V. Pierroz, K. Ingram, M. Kaiser, S. Ferrari, B. Spingler, J. Keiser, G. Gasser, Chem. A Eur. J. 19, 14768 (2013)
R. Gandhaveeti, R. Konakanchi, P. Jyothi, N.S.P. Bhuvanesh, S. Anandaram, Appl. Organomet. Chem. 33, e4899 (2019)
M.E. Reichmann, S.A. Rice, C.A. Thomas, P. Doty, J. Am. Chem. Soc. 76, 3047 (1954)
S. Nikolić, D.M. Opsenica, V. Filipović, B. Dojčinović, S. Arandelović, S. Radulović, S. Grgurić-Šipka, Organometallics 34, 3464 (2015)
D. Gopalakrishnan, M. Ganeshpandian, R. Loganathan, N.S.P. Bhuvanesh, X.J. Sabina, J. Karthikeyan, RSC Adv. 7, 37706 (2017)
M. Muralisankar, R. Dheepika, J. Haribabu, C. Balachandran, S. Aoki, N.S.P. Bhuvanesh, S. Nagarajan, ACS Omega 4, 11712 (2019)
D. Tran Buu, V. Duong Ba, M. Khoi Nguyen Hoang, T. Vu Quoc, L. Duong Khanh, Y. Oanh Doan Thi, L. Van Meervelt, Acta Crystallogr Sect. E Crystallogr. Commun. 75, 1389 (2019)
A.M. Pyle, J.P. Rehmann, R. Meshoyrer, N.J. Turro, J.K. Barton, C.V. Kumar, J. Am. Chem. Soc. 111, 3051 (1989)
M. Muralisankar, N.S.P. Bhuvanesh, A. Sreekanth, New J. Chem. 40, 2661 (2016)
X.Z. Feng, Z. Lin, L.J. Yang, C. Wang, C. Li Bai, Talanta 47, 1223 (1998)
M. Muralisankar, S.M. Basheer, J. Haribabu, N.S.P. Bhuvanesh, R. Karvembu, A. Sreekanth, Inorg. Chim. Acta 466, 61 (2017)
K.S. Ghosh, B.K. Sahoo, D. Jana, S. Dasgupta, J. Inorg. Biochem. 102, 1711 (2008)
K. Jeyalakshmi, J. Haribabu, C. Balachandran, N.S.P. Bhuvanesh, N. Emi, R. Karvembu, New J. Chem. 41, 2672 (2017)
E. Ramachandran, D. Senthil Raja, N.S.P. Bhuvanesh, K. Natarajan, Dalton Trans. 41, 13308 (2012)
P. Krishnamoorthy, P. Sathyadevi, R.R. Butorac, A.H. Cowley, N.S.P. Bhuvanesh, N. Dharmaraj, Dalton Trans. 41, 6842 (2012)
J.R. Lakowicz, G. Weber, Biochemistry 12, 4161 (1973)
K.N. Aneesrahman, G. Rohini, N.S.P. Bhuvanesh, S. Sundararaj, M. Musthafa, A. Sreekanth, ChemistrySelect 3, 8118 (2018)
P. Sathyadevi, P. Krishnamoorthy, R.R. Butorac, A.H. Cowley, N. Dharmaraj, Metallomics 4, 498 (2012)
Y.C. Huang, J. Haribabu, C.M. Chien, G. Sabapathi, C.K. Chou, R. Karvembu, P. Venuvanalingam, W.M. Ching, M.L. Tsai, S.C.N. Hsu, J. Inorg. Biochem. 194, 74 (2019)
M.A. Bhat, S.H. Lone, S. Ali, S.K. Srivastava, J. Mol. Struct. 1171, 233 (2018)
M. Musthafaa, R. Konakanchib, R. Gangulyc, A. Sreekanth, Phosphorus Sulfur Silicon Relat. Elem. 195, 331 (2020)
J. Prashanth, R. Konakanchi, B.V. Reddy, Mol. Simul. 45, 1353 (2019)
S.H. Lone, S. Jameel, M.A. Bhat, R.A. Lone, R.J. Butcher, K.A. Bhat, RSC Adv. 8, 8259 (2018)
K. Ramaiah, J. Prashanth, J. Haribabu, E. Sreekanth, B.V. Reddy, R. Karvembu, K.L. Reddy, J. Mol. Struct. 1175, 769 (2019)
R. Konakanchi, J. Haribabu, J. Prashanth, V.B. Nishtala, R. Mallela, S. Manchala, D. Gandamalla, R. Karvembu, B.V. Reddy, N.R. Yellu, L.R. Kotha, Appl. Organometal. Chem. 32, e4415 (2018)
G. Munikumari, R. Konakanchi, V.B. Nishtala, G. Ramesh, L.R. Kotha, K.B. Chandrasekhar, C. Ramachandraiah, Synth. Commun. 49, 146 (2019)
K. Ramaiah, K. Srishailam, K.L. Reddy, B.V. Reddy, G.R. Rao, J. Mol. Struct. 1184, 405 (2019)
R. Mallela, R. Konakanchi, R. Guda, N. Munirathinam, D. Gandamalla, N.R. Yellu, L.R. Kotha, Inorgan. Chim. Acta 469, 66 (2018)
R. Konakanchi, R. Mallela, R. Guda, L.R. Kotha, Res. Chem. Intermed. 44, 27 (2018)
T. Arun, S. Packianathan, M. Malarvizhi, R. Antony, N. Raman, J. Photochem. Photobiol. B Biol. 149, 93 (2015)
G. Moustafa, H. Khalaf, A. Naglah, A. Al-Wasidi, N. Al-Jafshar, H. Awad, Molecules 23, 1 (2018)
M. Musthafa, K.N. Aneesrahman, B. Perumalsamy, T. Ramasamy, R. Ganguly, A. Sreekanth, J. Mol. Struct. 1180, 585 (2019)
P. Mandal, B.K. Kundu, K. Vyas, V. Sabu, A. Helen, S.S. Dhankhar, C.M. Nagaraja, D. Bhattacherjee, K.P. Bhabak, S. Mukhopadhyay, Dalton Trans. 47, 517 (2018)
K.S. Neethu, J. Eswaran, M. Theetharappan, B. Nattamai, M.A. Neelakantan, K.M. Velusamy, Appl. Organomet. Chem. 33, 1 (2019)
G.M. Sheldrick SADABS, Program for Absorption Correction of Area Detector Frames, BRUKER AXS Inc., 5465 East Cheryl Parkway, Madison, USA
G.M. Sheldrick, Acta Crystallogr. Sect. A Found. Crystallogr. 64, 112 (2008)
Acknowledgements
The author Moideen Musthafa is thankful to MHRD, Govt. of India for providing Senior Research Fellowship and Ramaiah Konakanchi, and thanks are due to the Malla Reddy Engineering College for Women (Autonomous Institution), Hyderabad, India, for support and encouragement during this research work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Musthafa, M., Konakanchi, R., Ganguly, R. et al. Synthesis, characterization, in silico and in vitro biological activity studies of Ru(II) (η6-p-cymene) complexes with novel N-dibenzosuberene substituted aroyl selenourea exhibiting Se type coordination. Res Chem Intermed 46, 3853–3877 (2020). https://doi.org/10.1007/s11164-020-04177-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-020-04177-w