Abstract
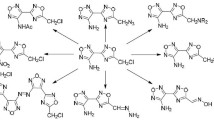
Nitro-, nitroso-, and azo-1,2,5-oxadiazoles with 4-R1-5-R2-1,2,3-triazol-1-yl substituents were synthesized by oxidation of amino-(1,2,3-triazol-1-yl)-1,2,5-oxadiazoles (aminotriazolylfurazans). Azido-1,2,5-oxadiazole was prepared by diazotization of amino(triazolyl)furazan followed by treatment of the diazonium salt with sodium azide. Depending on the nature of the substituents and the reagent, triazolylfurazans can undergo destruction to give amino-R-furazans (R = NO2, N3, aminofurazanylazo), the amino group being formed from the triazole ring.
Similar content being viewed by others
References
L. V. Batog, L. S. Konstantinova, V. Yu. Rozhkov, Yu. A. Strelenko, O. V. Lebedev, and L. I. Khmel'nitskii, Khim. Geterotsikl. Soedinen., 2000, 100 [Chem. Heterocycl. Compd., 2000, 36, 91 (Engl. Transl.)].
L. V. Batog, V. Yu. Rozhkov, Yu. A. Strelenko, O. V. Lebedev, and L. I. Khmel'nitskii, Khim. Geterotsikl. Soedinen., 2000, 406 [Chem. Heterocycl. Compd., 2000, 36, 343 (Engl. Transl.)].
L. V. Batog, V. Yu. Rozhkov, and M. I. Struchkova, Mendeleev Commun., 2002, 159.
L. V. Batog, V. Yu. Rozhkov, Yu. V. Khropov, N. V. Pyatakova, O. G. Busygina, I. S. Severina, and N. N. Makhova, Russ. Pat. No. 2158265 of 15.02.2000; Byul. izobret., 2000, No. 30, 167.
M. D. Coburn, J. Heterocycl. Chem., 1968, 83.
T. S. Novikova, T. M. Melnikova, O. V. Kharitonova, V. O. Kulagina, N. S. Aleksandrova, A. B. Sheremetev, T. S. Pivina, L. I. Khmelnitskii, and S. S. Novikov, Mendeleev Commun., 1994, 138.
R. Calvino, V. Mortarini, and A. Gasco, Eur. J. Med. Chem. —Chim. Ther., 1980, 15, 485.
G. D. Solodyuk, M. D. Boldyrev, B. V. Gidaspov, and V. D. Nikolaev, Zhurn. Organ. Khim., 1981, 17, 861 [J. Org. Chem. USSR, 1981, 17 (Engl. Transl.)].
A. B. Sheremetev, T. S. Novikova, T. M. Mel'nikova, and L. I. Khmel'nitskii, Izv. Akad. Nauk SSSR. Ser. Khim., 1990, 1193 [Bull. Acad. Sci. USSR, Div. Chem. Sci., 1990, 39, 1073 (Engl. Transl.)].
S. E. Semenov, A. M. Churakov, C. L. Ioffe, E. A. Vinogradova, S. G. Zlotin, and O. A. Luk'yanov, Izv. Akad. Nauk SSSR. Ser. Khim., 1991, 1940 [Bull. Acad. Sci. USSR, Div. Chem. Sci., 1991, 40, 1727 (Engl. Transl.)].
A. B. Sheremetev and O. V. Kharitonova, Mendeleev Commun., 1992, 157.
M. A. Epishina, N. N. Makhova, L. V. Batog, L. S. Konstantinova, and L. I. Khmelnitskii, Mendeleev Commun., 1994, 102.
L. V. Batog, L. S. Konstantinova, O. V. Lebedev, and L. I. Khmelnitskii, Mendeleev Commun., 1996, 193.
L. V. Batog, V. Yu. Rozhkov, L. S. Konstantinova, V. E. Eman, M. O. Dekaprilevich, Yu. T. Struchkov, S. E. Semenov, O. V. Lebedev, and L. I. Khmel'nitskii, Izv. Akad. Nauk. Ser. Khim., 1996, 1250 [Russ. Chem. Bull., 1996, 45, 1189 (Engl. Transl.)].
O. A. Rakitin, O. A. Zalesova, A. S. Kulikov, N. N. Makhova, T. I. Godovikova, and L. I. Khmel'nitskii, Izv. Akad. Nauk. Ser. Khim., 1993, 1949 [Russ. Chem. Bull., 1993, 42, 1865 (Engl. Transl.)].
B. Stanovnik, M. Tisler, S. Polanc, and Yu. Zitnik, Synthesis, 1977, 491.
H. E. Fierz-David and L. Blangey, Grundlegende Operationen der Farbenchemie, Springer Verlag, Wien, 1952, 244.
Author information
Authors and Affiliations
Additional information
__________
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 8, pp. 1859–1865, August, 2005.
Rights and permissions
About this article
Cite this article
Batog, L.V., Konstantinova, L.S. & Rozhkov, V.Y. Synthesis of nitro, nitroso, azo, and azido derivatives of (4-R1-5-R2-1,2,3-triazol-1-yl)-1,2,5-oxadiazoles by oxidation and diazotization of the corresponding amines. Russ Chem Bull 54, 1915–1922 (2005). https://doi.org/10.1007/s11172-006-0058-9
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s11172-006-0058-9