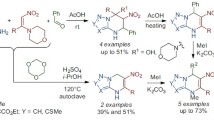
Abstract
A method of preparation of 5-amino-3,4-dinitropyrazole (1) from 3(5)-methyl-5(3)-nitro- and 3(5)-methyl-4,5(3)-dinitropyrazoles was developed, the key step of which was the Hofmann rearrengement of nitro- and dinitropyrazolecarboxamides. The protonation of 5-amino- 3,4-dinitropyrazole was studied by spectral methods (UV spectroscopy, NMR spectroscopy). In spite of low basicity of the amino group, compound 1 undergoes N-arylation, N-nitration, and annulation reactions with formation of dinitropyrazolo[5,1-a]pyrimidine derivatives and hitherto unknown dinitroimidazo[1,2-b]pyrazole derivatives. Diazotization of 1 leads to 5-diazo-3,4-dinitropyrazole (19), which exists in the form of the internal salt. Some reactions of this compound were studied and the formation of the corresponding 5-halogeno(azido)- 3,4-dinitropyrazoles under the action of the halide and azide ion was shown. Dinitropyrazolo- [5,1-c][1,2,4]triazine and 7-hydroxydinitro-4,7-dihydropyrazolo[5,1-c][1,2,4]triazine deriva- tives were obtained by the action of active methylene compounds on the betaine 19.
Similar content being viewed by others
|References
A. A. Zaitsev, D. V. Zaiko, I. L. Dalinger, V. V. Kachala, T. K. Shkineva, S. A. Shevelev, Izv. Akad. Nauk, Ser. Khim., 2009, 2058 [Russ. Chem. Bull., Int. Ed., 2009, 58, 2122].
I. L. Dalinger, S. A. Shevelev, Zh. Org. Khim., 1998, 34, 1127 [Russ. J. Org. Chem. (Engl Transl.), 1998, 34, 1071]; V. P. Lebedev, Yu. N. Matyushin, Ya. O. Inozemtcev, I. L. Dalinger, S. A. Shevelev, I. V. Fomenkov, Proc. 29th Intern. Ann. Conf. of ICT, Karlsruhe, FRG, 1998, 180.
I. L. Dalinger, T. I. Cherkasova, G. P. Popova, T. K. Shkineva, I. A. Vatsadse, S. A. Shevelev, M. I. Kanishchev, Izv. Akad. Nauk, Ser. Khim., 2009, 404 [Russ. Chem. Bull., Int. Ed., 2009, 58, 410].
S. A. Shevelev, I. L. Dalinger, T. K. Shkineva, B. I. Ugrak, V. I. Gulevskaya, M. I. Kanishchev, Izv. Akad. Nauk, Ser. Khim., 1993, 1108 [Russ. Chem. Bull. (Engl. Transl.), 1993, 42, 1063].
S. A. Shevelev, V. M. Vinogradov, I. L. Dalinger, T. I. Cherkasova, Izv. Akad. Nauk, Ser. Khim., 1993, 1945 [Russ. Chem. Bull. (Engl. Transl.), 1993, 42, 1861].
Yu. A. Manaev, M. A. Andreeva, V. P. Perevalov, B. I. Stepanov, V. A. Dubrovskaya, V. I. Seraya, J. Obshch. Khim., 1982, 52, 2592 [J. Gen. Chem. USSR (Engl. Transl.), 1982, 52, No. 11].
P. Rzepecki, H. Gallmeier, N. Geib, K. Gernovska, T. Schrader, J. Org. Chem., 2004, 69, 5168; I. L. Dalinger, I. A. Vatsadse, S. A. Shevelev, A. V. Ivachtchenko, J. Comb. Chem., 2005, 7, 236; D. Dressen, A. W. Garofalo, J. Hawkinson, D. Hom, J. Jagodzinski, J. L. Marugg, M. L. Neitzel, M. A. Pleiss, B. Szoke, J. S. Tung, D. W. G. Wone, J. Wu, H. Zhang, J. Med. Chem., 2007, 50, 5161.
P. Rzepecki, M. Wehner, O. Molt, R. Zadmard, K. Harms, T. Schrader, Synthesis, 2003, 1815.
I. L. Dalinger, T. I. Cherkasova, S. A. Shevelev, Mendeleev. Commun., 1997, No. 2, 58.
J. W. A. M. Janssen, H. J. Koeners, C. G. Kruse, C. L. Habraken, J. Org. Chem., 1973, 38, 1777.
V. M. Vinogradov, T. I. Cherkasova, I. L. Dalinger, S. A. Shevelev, Izv. Akad. Nauk, Ser. Khim., 1993, 1616 [Russ. Chem. Bull. (Engl. Transl.), 1993, 42, 1552].
G. A. Morris, R. Freeman, J. Am. Chem. Soc., 1979, 101, 760.
K. G. R. Pachler, P. L. Pachler, J. Magn. Reson., 1973, 12, 377.
A. A. Zaitsev, I. L. Dalinger, S. A. Shevelev, Usp. Khim., 2009, 78, 643 [Russ. Chem. Rev. (Engl. Transl.), 2009, 78].
L. Larina, V. Lopyrev, Nitroazoles. Synthesis, Structure and Applications, Springer, New York, 2009, 196.
J. Catalan, J. L. Abband, J. Elquero, Adv. Het. Chem., 1987, 41, 250.
Author information
Authors and Affiliations
Corresponding author
Additional information
For Part 17, see Ref. 1.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 8, pp. 1589–1595, August, 2010.
Rights and permissions
About this article
Cite this article
Dalinger, I.L., Vatsadse, I.A., Shkineva, T.K. et al. Nitropyrazoles. Russ Chem Bull 59, 1631–1638 (2010). https://doi.org/10.1007/s11172-010-0287-9
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-010-0287-9
- Key words
- pyrazole
- dinitropyrazole
- 5-amino-3,4-dinitropyrazole
- 5-diazo-3,4-dinitro-pyrazole
- the Hofmann rearrangement
- N-arylation
- N-nitration
- annulation
- imidazo[1,2-b]- pyrazole
- pyrazolo[5,1-a]pyrimidine
- diazotization
- active methylene compounds
- pyrazolo-[5,1-c][1,2,4]triazine
- dihydropyrazolo[5,1-c][1,2,4]triazine