Abstract
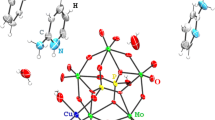
Crystalline compounds (H3O)2(phz)3M2(C6O4Cl2)3·(CH3COCH3) n ·(H2O) n (n = 0−2, M = Mn (1), Fe (3)) were obtained in an acetone-water-tetrahydrofuran medium by the reaction of metal sulfates with chloranilic acid and phenazine (phz). The molecular and crystal structure of 1 was studied by X-ray diffraction at 300, 200, and 150 K. The crystal structure is composed of polymeric cationic [(H3O)2(phz)3] n 2n+ and anionic [Mn2(C6O4Cl2)3] n 2n− layers having honeycomb structure and stacked in such a way that open-ended through-going channels accommodating H2O and CH3COCH3 solvent molecules are formed; the H3O+ cations in the crystal structure are disordered. Magnetic studies indicate antiferromagnetic coupling of Mn2+ ions through chloranilate ligands; transition to a magnetically ordered state occurs at T = 5 K. According to powder X-ray diffraction data, complex 3 is isostructural with compound 1 but differs crucially from 1 in the electronic structure. According to Fe57 Mössbauer spectroscopy, complex 3 exists in delocalized mixed-valence Fe2+/Fe3+ state and, as a consequence, shows ferromagnetic character of magnetic correlations and semiconductor type of electrical conductivity. These features were ascribed to the valence tautomerism Fe2+ + (C6O4Cl2)2− → Fe3+ + (C6O4Cl2)·3−, which was observed for the first time for iron in a honeycomb structure.
Similar content being viewed by others

References
V. I. Ovcharenko, R. Z. Sagdeev, Usp. Khim., 1999, 68, 381 [Russ. Chem. Rev. (Engl. Transl.), 1999, 68].
Z. J. Zhong, N. Matsumoto, H. Okawa, S. Kida, Chem. Lett., 1990, 87.
H. Tamaki, M. Mitsumi, K. Nakamura, N. Matsumoto, S. Kida, H. Okawa, S. Ijima, Chem. Lett., 1992, 1975.
H. Tamaki, Z.J. Zhong, N. Matsumoto, S. Kida, M. Koikawa, N. Achiva, Y. Nachimoto, H. Okawa, J. Am. Chem. Soc., 1992, 114, 6974.
L. O. Atovmyan, G. V. Shilov, R. N. Lyubovskaya, E. I. Zhilyaeva, N. S. Ovanesyan, S. I. Pirumova, I. G. Gusakovskaya, Pis’ma v Zh. Eksp. Teor. Fiz., 1993, 58, 818 [JETP Lett. (Engl. Transl.), 1993, 58, 766].
C. Mathoniere, S. G. Carling, Y. Dou, P. Day, Chem. Commun., 1994, 1551.
C. Mathoniere, C. J. Nutall, S. G. Carling, P. Day, Inorg. Chem., 1996, 35, 1201.
N. S. Ovanesyan, G. V. Shilov, L. O. Atovmyan, R. N. Lyubovskaya, A. A. Pyalling, Mol. Cryst. Liq. Cryst., 1995, 273, 175.
S. Decurtins, H. W. Schmalle, P. Schneuly, J. Ensling, P. Gutlich, J. Am. Chem. Soc., 1994, 116, 9521.
E. Coronado, J. R. Galan-Mascaros, C. G. Gomez-Garcia, E. Martinez-Ferrero, M. Almeida, Eur. J. Inorg. Chem., 2005, 2064.
S. Benard, P. Yu, J. P. Audiere, E. Riviere, R. Clement, J. Guilhem, L. Tchertanov, K. Nakatani, J. Am. Chem. Soc., 2000, 122, 9444.
E. Coronado, J. R. Galan-Mascaros., C. J. Gomez-Garcia, V. Laukhin, Nature (London), 2000, 408, 447.
M. Gruselle, C. Train, K. Boubekeur, P. Gredin, N. S. Ovanesyan, Coord. Chem. Rev., 2006, 250, 2491.
S. M. Aldoshin, L. A. Nikonova, G. V. Shilov, E. A. Bikanina, N. K. Artemova, V. A. Smirnov, Mol. Struct., 2006, 794, 103.
C. G. Pierpont, L. C. Francesconi, D. H. Hendickson, Inorg. Chem., 1977, 16, 2367.
D. F. Xiang, C. Y. Duan, X. S. Tan, Y. J. Liu, W. X. Tang, Polyhedron, 1998, 17, 2647.
M. Kawahara, M. K. Kabir, K. Yamada, K. Adachi, H. Kumagai, Y. Narumi, K. Kindo, S. Kitagawa, S. Kawata, Inorg. Chem., 2004, 43, 92.
A. Dei, D. Gatteschi, L. Pardi, U. Russo, Inorg. Chem., 1991, 30, 2589.
K. S. Min, A. G. DiPasquale, J. A. Golen, A. L. Rheingold, J. S. Miller, J. Am. Chem. Soc., 2007, 129, 2360.
J. T. Wrobleski, D. B. Brown, Inorg. Chem., 1979, 18, 498.
S. Kawata, S. Kitagawa, H. Kumagai, T. Ishiyama, K. Honda, H. Tobita, K. Adachi, M. Katada, Chem. Mater., 1998, 10, 3902.
B. F. Abrahams, K. D. Lu, B. Moubaraki, K. S. Murray, R. Robson, J. Chem. Soc., Dalton Trans., 2000, 1793.
S. Kawata, S. Kitagawa, I. Furuchi, C. Kudo, H. Kamesaki, M. Kondo, M. Katada, M. Munakata, Mol. Cryst. Liq. Cryst., 1995, 274, 179.
S. Kawata, S. Kitagawa, M. Kondo, I. Furuchi, M. Munakata, Angew. Chem., Int. Ed., 1994, 33, 1759.
H. Kumagai, S. Kawata, S. Kitagawa, Inorg. Chim. Acta, 2002, 337, 387.
M. K. Kabir, M. Kawahara, H. Kumagai, K. Adachi, S. Kawata, T. Ishii, S. Kitagawa, Polyhedron, 2001, 20, 1417.
S. Kawata, S. Kitagawa, H. Kumagai, C. Kudo, H. Kamaesaki, T. Ishiyama, R. Suzuki, M. Kondo, M. Katada, Inorg. Chem., 1996, 35, 4449.
M. K. Kabir, S. Kawata, K. Adachi, H. Tobita. N. Miyazaki, H. Kumagai, M. Katada, S. Kitagawa, Mol. Cryst. Liq. Cryst., 2000, 341, 491.
K. Nagayoshi, M. D. Kabir, H. Tobita, K. Honga, M. Kawahara, M. Katada, K. Adachi, H. Nishikawa, I. Ikemoto, H. Kumagai, Y. Hosokoshi, K. Inoue, S. Kitagawa, S. Kawata, J. Am. Chem. Soc., 2003, 125, 221.
Tzuoo-Tsair Luo, Yen-Hsiang Liu, Hui-Lien Tsai, ChanCheng Su, Chuen-Her Ueng, Kuang-Lieh Lu, Eur. J. Inorg. Chem., 2004, 4253.
P. E. Riley, S. F. Haddad, K. N. Raymond, Inorg. Chem., 1983, 22, 3090.
B. F. Abrahams, J. Coleiro, K. Ha, B. F. Hoskins, S. D. Orehard, R. Robson, J. Chem. Soc., Dalton Trans., 2002, 1586.
Z. K. Nikitina, V. D. Makhaev, N. S. Ovanesyan, G. V. Shilov, S. M. Aldoshin, Int. Conf. Organometallic and Coord Chem. (Russia, N. Novgorod, 2008, 2–8 September), Book of Abstracts, N. Novgorod, 2008, 74.
Z. K. Nikitina, N. S. Ovanesyan, V. D. Makhaev, G. V. Shilov, S. M. Aldoshin, Dokl. Akad. Nauk, 2011, 437, 778 [Dokl. Chem. (Engl. Transl.), 2011, 437, Pt. 2, 129].
G. M. Sheldrick, SHELXTL v. 6.14, Structure Determination Software Suite, Bruker AXS, Madison, Wisconsin, USA, 2000.
K. P. Butin, E. K. Beloglazkina, N. V. Zyk, Usp. Khim., 2005, 74, 585 [Russ. Chem. Rev. (Engl. Transl.), 2005, 74].
K. S. Min, A. L. Reingold, J. S. Miller, J. Am. Chem. Soc., 2006, 128, 40.
X.-Q. Ding, E. L. Bominar, E. Bill, H. Winkler, A. X. Trautwein, S. Drueke, P. Chaudhuri, K. Wieghardt, J. Chem. Phys., 1990, 92, 178.
D. Lee, C. Krebs, B. H. Huynh, M. P. Hendrich, S. J. Lippard, J. Am. Chem. Soc., 2000, 122, 5000.
X.-Q. Ding, E. Bill, A. X. Trautwein, H. Winkler, A. Kostikas, V. Papaefthymiou, A. Simopoulos, P. Beardwood, J. F. Gibson, Chem. Phys., 1993, 99, 6421.
C. Zener, Phys. Rev., 1951, 81, 403.
P. W. Anderson, H. Hasegawa, Phys. Rev., 1955, 100, 675.
G. H. Jonker, J. H. Van Santen, Physica, 1950, 16, 599.
J. S. Miller, K. S. Min, Angew. Chem., Int. Ed., 2008, 47, 2.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 6, pp. 1183–1193, June, 2011.
Rights and permissions
About this article
Cite this article
Shilov, G.V., Nikitina, Z.K., Ovanesyan, N.S. et al. Phenazineoxonium chloranilatomanganate and chloranilatoferrate: synthesis, structure, magnetic properties, and Mössbauer spectra. Russ Chem Bull 60, 1209–1219 (2011). https://doi.org/10.1007/s11172-011-0190-z
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-011-0190-z