Abstract
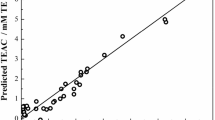
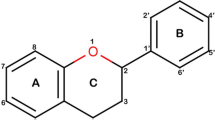
The quantitative structure-activity relationship of a set of 19 flavonoid compounds presenting antioxidant activity was studied by means of PLS (Partial Least Squares) regression. The optimization of the structures and calculation of electronic properties were done by using the semiempirical method AM1. A reliable model (r 2=0.806 and q 2=0.730) was obtained and from this model it was possible to consider some aspects of the structure of the flavonoid compounds studied that are related with their free radical scavenging ability. The quality of the PLS model obtained in this work indicates that it can be used in order to design new flavonoid compounds that present ability to scavenge free radicals.




Similar content being viewed by others
References
Kawanishi S, Hiraku Y, Oikawa S (2001) Mutat Res 488:65–76
Khan MA, Baseer A (2001) J Pak Med Assoc 50:261–264
Warma SD, Devamanoharan PS, Morris SM (1995) Crit Rev Food Sci Nutr 35:111–129
Halliwell B (1994) Lancet 344:721–724
Block G, Patterson B, Subar A (1992) Nutr Cancer 17:1–29
Gillman MW, Cupples LA, Gagnon D, Posner BM, Ellison C, Castelli WP, Wolf P (1995) J Am Med Assoc 273:1113–1117
Peterson J, Dwyer J (1998) Nutr Res 12:1995–2018
Block G, Langseth L (1994) Food Technol 80–84
Block G (1992) Nutr Rev 50:207–213
Hertog MGL, Feskens EJM, Hollman PCH, Katan MB, Kromhout D (1993) Lancet 342:1007–1011
Kuhnau J (1976) World Rev Nutr Diet 11:433–448
Pietta P-G (2000) J Nat Prod 63:1035–1042
Rice-Evans CA, Miller NJ, Paganga G (1996) Free Radical Biol Med 20:933–956
van Acker SABE, van den Berg D-J, Tromp MNJL, Griffioen DH, Van Bennekom WP, van der Vijgh WJF, Bast A (1996) Free Radical Biol Med 20:331–342
Jovanovic SV, Steenken S, Hara Y, Simic MG (1996) J Chem Soc Perkin Trans 2:2497–2504
Cao G, Sofic E, Prior RL (1997) Free Radical Biol Med 22:749–760
Arora A, Nair MG, Strasburg GM (1998) Free Radical Biol Med 24:1355–1363
Yokozawa T, Chen CP, Dong E, Tanaka T, Nonaka G-I, Nishioka I (1998) Biochem Pharmacol 56:213–222
Heijnen CGM, Haenen GRMM, van Acker FAA, van der Vijgh WJF, Bast A (2001) Toxicol In Vitro 32:111–121
Weber KC, Honório KM, da Silva SL, Mercadante R, da Silva ABF (2005) Int J Quantum Chem 103:731–737
Lien EJ, Ren S, Bui H-H, Wang R (1999) Free Radical Biol Med 26:285–294
Farkas O, Jakus J, Heberger K (2004) Molecules 9:1079–1088
Rasulev BF, Abdullaev ND, Syrov VN, Leszczynski J (2005) QSAR Comb Sci 1056–1065
van Acker FAA, Hageman JA, Haenen GRMM, van der Vijgh WJF, Bast A, Menge WMPB (2000) J Med Chem 43:3752–3760
Penga ZF, Strackb D, Baumertb A, Subramaniama R, Goha NK, Chiaa TF, Tana SN, Chiaa LS (2003) Phytochemistry 62:219–228
Ishige K, Schubert D, Sagara Y (2001) Free Radical Biol Med 30:433–446
Allinger NL, Yuh YH, Lin JH (1989) J Am Chem Soc 111:8551–8566
Ostlund NS (1995) ChemPlus: Program for molecular visualization and simulation. University of Waterloo, Canada
Dewar MJS, Zoebisch EG, Healy EF, Stewart JJP (1985) J Am Chem Soc 107:3902–3909
Semichem (1997) Ampac 6.5, Shawnee, OK
Zhang H-Y (1998) J Am Oil Chem Soc 75:1705–1709
Zhang H-Y (2000) Quant Struct–Act Relat 19:50–53
Todeschini R, Consonni V, Pavan M (2002) Dragon 2.1. Milan, Italy
Infometrix Inc. (2002) Pirouette 3.11, Woodinville, WA
Wold S, Sjöström M, Eriksson L (2001) Chemom Intell Lab Syst 58:109–130
Wold S, Ruhe A, Wold H, Dunn WJ, III (1984) SIAM J Sci Stat Comput 5:735–743
Heim KE, Tagliaferro AR, Bobilya DJ (2002) J Nutr Biochem 13:572–584
Kerry N, Rice-Evans C (1999) J Neurochem 73:247–253
Dugas AJ Jr, Castaneda-Acosta J, Bonin G, Price KL, Fischer NH, Winston GW (2000) J Nat Products 63:327–331
Sekher Pannala A, Chan TS, O’Brien PJ, Rice-Evans CA (2001) Biochem Biophys Res Commun 282:1161–1168
Sharaf MA, Illman DL, Kowalski BR (1986) Chemometrics. Wiley & Sons, New York, 357p
Weisberg S (1985) Applied linear regression. John Wiley & Sons, New York, Chapter 5
Clark RD, Foz PC (2004) J Comput Aid Mol Des 18:563–576
Papa E, Villa F, Gramatica P (2005) J Chem Inf Model 45:1256–1266
Acknowledgements
The authors acknowledge the financial support given by CAPES and FAPESP (Brazilian agencies).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Weber, K.C., Honório, K.M., Bruni, A.T. et al. A partial least squares regression study with antioxidant flavonoid compounds. Struct Chem 17, 307–313 (2006). https://doi.org/10.1007/s11224-006-9048-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-006-9048-7