Abstract
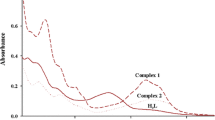
A Schiff base ligand containing thiocarbamide group of 4-phenyl-1-(4-methoxyl-1-phenylethylidene)thiosemicarbazide (HL) and its three mononuclear metal complexes of ZnL2 (1), NiL2 (2), and CuL2 (3) have been synthesized. Elemental analysis, IR, and X-ray single crystal diffraction characterizations for the ligand and the three complexes have been carried out. In the three complexes, the central metallic ions of Zn2+, Ni2+, and Cu2+ coordinate with two deprotonated ligands of L−, respectively. In 1, Zn2+ ion adopts a distorted tetrahedral geometry, while in 2 and 3, both the Ni2+ and Cu2+ ions possess distorted square planar configurations. For the four compounds, UV–Vis spectra have been measured and DFT calculations at B3LYP/LANL2DZ level of theory prove that the electronic spectra of HL and 1 are corresponding with electronic transitions of n → π* and π → π* in the ligand itself and the electronic spectra of 2 and 3 are attributed to intraligand electronic transitions as well as d–d electronic transitions. Electrochemical investigations reveal that the different metal–ligand interactions have changed the peak shapes and peak locations, which are corresponding with the DFT-B3LYP/LANL2DZ calculational results. Fluorescence spectra measurements indicate that the ligand emits purple fluorescence and the complex 1 emits stronger blue fluorescence, while the complexes 2 and 3 quench fluorescence. The thermal analyses result show that the three complexes undergo two similar decomposition processes because of their similar geometric configurations.






Similar content being viewed by others
References
Tao XT, Suzuki H, Wada T, Sasabe H, Miyata S (1999) Appl Phys Lett 75:1655
Seong NC, Jeon YM, Lim TH, Kim JW, Lee CW, Lee EJ, Jang JG, Jang HJ, Lee JY, Gong MS (2007) Synth Met 157(10–12):421
Kishigami Y, Tsubaki K, Kondo Y, Kido J (2005) Synth Met 153(1–3):241
Ho MH, Chang CM, Chu TY, Chen TM, Chen CH (2008) Org Electron 9(1):101
Wu YZ, Zheng XY, Zhu WQ, Sun RG, Jiang XY, Zhang ZL, Xu SH (2003) Appl Phys Lett 83:5077
Kasai K, Aoyagi M, Fujita M (2000) J Am Chem Soc 122(9):2140
Kitagawa S, Kitaura R, Noro SI (2004) Angew Chem Int Ed 43(18):2334
Hoshino N, Ito T, Nihei M, Oshio H (2003) Inorg Chem Commun 6(4):377
Khandar AA, Nejati K (2000) Polyhedron 19(6):607
Zhou HP, Li DM, Wang P, Cheng LH, Gao YH, Zhu YM, Wu JY, Tian YP, Tao XT, Jiang MH, Fun HK (2007) J Mol Struct 826(2–3):205
Hamada Y, Sano T, Fujita M, Fujii T, Nishio Y, Shibata K (1993) Jpn J Appl Phys 32:L511
Kim SM, Kim JS, Shin DM, Kim YK, Ha Y (2001) Bull Korean Chem Soc 22(7):743
Yu G, Liu YQ, Song Y, Wu X, Zhu D (2001) Synth Met 117(1–3):211
Yu TZ, Su WM, Li WL, Hong ZR, Hu RN, Li B (2007) Thin Solid Films 515(7–8):4080
Hui JKH, Yu Z, MacLachlan MJ (2007) Angew Chem Int Ed 46(42):7980
Wang HY, Zhao PS, Shao DL, Zhang J, Zhu YL (2009) Struct Chem 20(6):995
de Aquino TM, Liesen AP, da Silva REA, Lima VT, Carvalho CS, de Faria AR, de Araújo JM, de Lima JG, Alves AJ, de Melo EJT, Góes AJS (2008) Bioorg Med Chem 16:446
Cunha S, da Silva TL (2009) Tetrahedron Lett 50:2090
Vila JM, Pereira T, Amoedo A, Graña M, Martínez J, López-Torres M, Fernández A (2001) J Organomet Chem 623:176
SMART and SAINT for Windows NT Software Reference Mannuals, Version 5.0; Bruker Analytical X-Ray Systems: Madison, WI, 1997
Sheldrick GM (1997) SADABS-A software for empirical absorption correction. University of Göttingen, Göttingen, Germary
SHELXTL Reference Manual, Version 5.1; Bruker Analytical X-Ray Systems: Madison, WI, 1997
Peng C, Ayala PY, Schlegel HB, Frisch MJ (1996) J Comput Chem 17(1):49
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Vreven T Jr, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian, Inc., Gaussian 03, Revision C.01, Wallingford, CT
Runge E, Gross EKU (1984) Phys Rev Lett 52(12):997
Petersilka M, Gossmann UJ, Gross EKU (1966) Phys Rev Lett 76(8):1212
Jian FF, Li Y, Xiao HL (2005) Acta Crystallogr E 61:o2219
de Sousa GF, West DX, Brown CA, Swearingen JK, Valdés-Martínez J, Toscano RA, Hernández-Ortega S, Hörner M, Bortoluzzi AJ (2000) Polyhedron 19(7):841
Liu ZH, Duan CY, Li JH, Liu YJ, Mei YH, You XZ (2000) New J Chem 24:1057
Ali MA, Mirza AH, Bucher RJ, Rahman M (2000) Transition Met Chem 25(4):430
Jouad EM, Allain M, Khan MA, Bouet GM (2005) Polyhedron 24(2):327
Wang J (2000) Analytical Electrochemistry, 2nd edn. Wiley-VCH, New York
Xu ZC, Xiao Y, Qian XH, Cui JN, Cui DW (2005) Org Lett 7(5):889
Acknowledgement
This work was supported by Huaian Science & Technology Bureau, Jiangsu Province, P. R. China (HAG09054-7, HAC0804), Fund of Huanyin Teachers College (07HSBS004, 08HSJSK003), and Fund of Jiangsu Key Laboratory for Chemistry of Low-Dimensional Materials (JSKC08047).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, P.S., Wang, H.Y., Song, J. et al. Synthesis, structures, and property studies on Zn(II), Ni(II), and Cu(II) complexes with a Schiff base ligand containing thiocarbamide group. Struct Chem 21, 977–987 (2010). https://doi.org/10.1007/s11224-010-9634-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-010-9634-6