Abstract
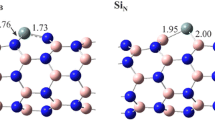
Adsorption of toxic hydrazine (N2H4) at the surface of pristine and Al-doped single-wall boron nitride nanotubes (BNNTs and Al-BNNTs) has been investigated using density functional theory (DFT). Single hydrazine molecule was allowed to interact with Lewis acid center (i.e., B atom) of BNNTs, where most stable gauche conformer of the guest is used. Both zigzag (8,0) and armchair (4,4) variants of BNNTs were considered and to estimate the effect of tube diameter on adsorption energies wider zigzag (9,0) and (10,0), and armchair (5,5) and (6,6), tubes were included in the host list. Estimated adsorption energies of 2–8 kcal/mol do not support strong binding between pristine BNNTs with hydrazine. However, Al doping makes BNNTs an attractive adsorbent of N2H4 with adsorption energy in the range of 32–35 kcal/mol. Stability of such complexes is found less sensitive to the chirality, but nominally decreases with tube diameter. Thus, a large quantity of hydrazine may be adsorbed by Al-BNNT samples. The dative N: → B/Al bond between hydrazine and host tubes is the source of stability of complexes. Molecular electrostatic potentials (MEPs) and frontier molecular orbitals of host and guest were calculated to explain interaction character and strength.




Similar content being viewed by others
References
Agency UEP (1999) Integrated Risk Information System (IRIS) on hydrazine/hydrazine sulfate. National Center for Environmental Assessment, Office of Research and Development, Washington, DC
Reilly CA, Aust S (1997) Peroxidase substrates stimulate the oxidation of hydralazine to metabolites which cause single-strand breaks in DNA. Chem Res Toxicol 10(3):328–334
Zelnick SD, Mattie DR, Stepanial PC (2003) Occupational exposure to hydrazines: treatment of acute central nervous system toxicity. Abviat Space Environ Med 74(12):1285–1291
Garrod S, Bollard ME, Nicholls AW, Connor SC, Connelly J, Nicholson JK, Holmes E (2005) Integrated metabonomic analysis of the multiorgan effects of hydrazine toxicity in the rat. Chem Res Toxicol 18(2):115–122
Ragnarsson U (2001) Synthetic methodology for alkyl substituted hydrazines. Chem Soc Rev 30:205–213
Narayanan SS, Scholz F (1999) A comparative study of the electrocatalytic activities of some metal hexacyanoferrates for the oxidation of hydrazine. Electroanalysis 11:465–469
Yamada K, Yasuda K, Fujiwara N, Siroma Z, Tanaka H, Miyazaki Y, Kobayashi T (2003) Potential application of anion-exchange membrane for hydrazine fuel cell electrolyte. Electrochem Commun 5(10):892–896. https://doi.org/10.1016/j.elecom.2003.08.015
Singh SK, Xu Q (2013) Nanocatalysts for hydrogen generation from hydrazine. Catal Sci Technol 3:1889–1900
Pelegrini M, Parreira RLT, Ferrão LFA, Caramori GF, Ortolan AO, da Silva EH, Roberto-Neto O, Rocco JAFF, Machado FBC (2016) Hydrazine decomposition on a small platinum cluster: the role of N2H5 intermediate. Theo Chem Acc 135(3):58
Asazawa K, Sakamoto T, Yamaguchi S, Yamada K, Fujikawa H, Tanaka H, Oguro K (2009) Study of anode catalysts and fuel concentration on direct hydrazine alkaline anion-exchange membrane fuel cells. J Electrochem Soc 156(4):B509–B512
Yang H, AZhong X, Dong Z, Wang J, Jin J, Ma J (2012) A highly active hydrazine fuel cell catalyst consisting of a Ni–Fe nanoparticle alloy plated on carbon materials by pulse reversal. RSC Adv 2:5038–5040
Sutton GP, Biblarz O (2010) Rocket propulsion elements, 8th edn. Wiley, New York,
Agusta MK, Kasai H (2012) First principles investigations of hydrazine adsorption conformations on Ni(111) surface. Surf Sci 606:766–771
He YB, Jia JF, Wu HS (2015) The interaction of hydrazine with an Rh(1 1 1) surface as a model for adsorption to rhodium nanoparticles: a dispersion-corrected DFT study. Appl Sutf Sci 327:462–469
He Y-B, Jia J-F, Wu H-S (2015) Selectivity of Ni-based surface alloys toward hydrazine adsorption: a DFT study with van der Waals interactions. Appl Surf Sci 339:36–45
Fathurrahman F, Kasai H (2015) Density functional study of hydrazine adsorption and its N=N bond cleaving on Fe(110) surface. Surf Sci 639:25–31
Daemi S, Ashkarran AA, Bahari A, Ghasemi S (2017) Fabrication of a gold nanocage/graphene nanoscale platform for electrocatalytic detection of hydrazine. Sensors Actuators B Chem 245:55–65
Hao Y, Zhang Y, Ruan K, Chen W, Zhou B, Tan X, Wang Y, Zhao L, Zhang G, Qu P, Xu M (2017) A naphthalimide-based chemodosimetric probe for ratiometric detection of hydrazine. Sensors Actuators B Chem 244:417–424
Zhang J, Gao W, Dou M, Wang F, Liu J, Li Z, Ji J (2015) Nanorod-constructed porous Co3O4 nanowires: highly sensitive sensors for the detection of hydrazine. Analyst 140:1686–1692
Deng K, Li C, Qiu X, Zhou J, Hou Z (2015) Synthesis of cobalt hexacyanoferrate decorated graphene oxide/carbon nanotubes-COOH hybrid and their application for sensitive detection of hydrazine. Electrochim Acta 174:1096–1103
Tafreshi SS, Roldan A, Dzade NY, de Leeuw NH (2014) Adsorption of hydrazine on the perfect and defective copper (111) surface: a dispersion-corrected DFT study. Surf Sci 622:1–8
Esrafili MD, Teymurian VM, Nurazar R (2015) Catalytic dehydrogenation of hydrazine on silicon-carbide nanotubes: a DFT study on the kinetic issue. Surf Sci 632:118–125
Zhang P-X, Wang Y-G, Huang Y-Q, Zhang T, Wu G-S, Li J (2011) Density functional theory investigations on the catalytic mechanisms of hydrazine decompositions on Ir(1 1 1). Catal Today 165:80–88
McKay HL, Jenkins SJ, Wales DJ (2011) Dissociative chemisorption of hydrazine on an Fe{2 1 1} surface. J Phys Chem C 115:17812–17828
Terrones M, Hsu WK, Terrones H, Zhang JP, Ramos S, Hare JP, Castillo R, Prasside K, Cheetham AK, Kroto HW, Walton DRM (1996) Metal particle catalyzed production of nanoscale BN structures. Chem Phys Lett 259:568–573
Loiseau A, Willaime F, Demoncy N, Hug G, Pascard H (1996) Boron nitride nanotubes with reduced numbers of layers synthesized by arc discharge. Phys Rev Lett 76(25):4737–4740
Terauchi M, Tanaka M, Takehisa M, Saito Y (1998) Electron energy-loss spectroscopy study of the electronic structure of boron nitride nanotubes. J Electron Microsc 47(4):319–324
Golberg D, Bando Y, Eremets M, Takemura K, Kurashima K, Tamiya K, Yusa H (1997) Boron nitride nanotube growth defects and their annealing-out under electron irradiation. Chem Phys Lett 279(3–4):191–196
Laude T, Matsui Y, Marraud A, Jouffrey B (2000) Long ropes of boron nitride nanotubes grown by a continuous laser heating. App Phys Lett 76(22):3239–3241
Golberg D, Bando Y, Sato T, Grobert N, Reyes-Reyes M, Terrones H, Terrones M (2002) Nanocages of layered BN: super-high pressure nanocells for formation of solid nitrogen. J Chem Phys 116(9):8523–8532
Saha S, Dinadayalane TC, Leszczynska D, Leszczynski J (2012) Open and capped (5,5) armchair SWCNTs: a comparative study of DFT-based reactivity descriptors. Chem Phys Lett 541:85–91
Saha S, Dinadayalane TC, Murray JS, Leszczynska D, Leszczynski J (2012) Surface reactivity for chlorination on chlorinated (5,5) armchair SWCNT: a computational approach. J Phys Chem C 116:22399–22410
Saha S, Dinadayalane TC, Leszczynska D, Leszczynski J (2013) DFT-based reactivity study of (5,5) armchair boron nitride nanotube (BNNT). Chem Phys Lett 565:69–73
Garel J, Leven I, Zhi C, Nagapriya KS, Popovitz-Biro R, Golberg D, Bando Y, Hod O, Joselevich E (2012) Ultrahigh torsional stiffness and strength of boron nitride nanotubes. Nano Lett 12:6347–6352
Huang Y, Lin J, Zou J, Wang M-S, Faerstein K, Tang C, Bando Y, Golberg D (2013) Thin boron nitride nanotubes with exceptionally high strength and toughness. Nano 5:4840–4846
Sundaram R, Scheiner S, Roy AK, Kar T (2015) Site and chirality selective chemical modifications of boron nitride nanotubes (BNNTs) via Lewis acid/base interactions. Phys Chem Chem Phys 17:3850–3866
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Sundaram R, Scheiner S, Roy AK, Kar T (2015) B═N bond cleavage and BN ring expansion at the surface of boron nitride nanotubes by iminoborane. J Phys Chem C 119:3253–3259
Wu X, An W, Zeng XC (2006) Chemical functionalization of boron-nitride nanotubes with NH3 and amino functional groups. J Am Chem Soc 128:12001–12006
Li Y, Zhou Z, Zhao J (2007) Transformation from chemisorption to physisorption with tube diameter and gas concentration: computational studies on NH3 adsorption in BN nanotubes. J Chem Phys 127:184705–184706
Ahmadi A, Beheshtian J, Hadipour NL (2011) Chemisorption of NH3 at the open ends of boron nitride nanotubes: a DFT study. Struct Chem 22:183–188
Zahedi E (2012) Effect of tube radius on the exohedral chemical functionalization of boron-nitride zigzag nanotubes with NH3. Physica B 407:3841–3848
Rimola A, Sodupe M (2013) Physisorption vs. chemisorption of probe molecules on boron nitride nanomaterials: the effect of surface curvature. Phys Chem Chem Phys 15:13190–13198
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Jaramillo J, Stratmann RE, Yazyev O, Austin A, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian09. Gaussian, Inc., Wallingford,
Soltani A, Raz SG, Rezaei VJ, Dehno Khalaji A, Savar M (2012) Ab initio investigation of Al- and Ga-doped single-walled boron nitride nanotubes as ammonia sensor. Appl Surf Sci 263:619–625
Azizi K, Karimpanah M (2013) Computational study of Al- or P-doped single-walled carbon nanotube as NH3 and NO2 sensors. Appl Surf Sci 285P:102–109
Mulliken RS (1955) Electronic population analysis on LCAO-MO molecular wave functions. I. J Chem Phys 23(10):1833–1840
Mulliken RS (1955) Electron population analysis on LCAO-MO molecular wave functions. II. Overlap populations, bond orders, and bond energies. J Chem Phys 23(10):1841–1846
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88:899–926
Reed AE, Weinhold F, Curtiss LA, Pochatko DJ (1986) Natural bond orbital analysis of molecular interactions: theoretical studies of binary complexes of HF, H2O, NH3, N2, O2, F2, CO, and CO2 with HF, H2O, and NH3. J Chem Phys 84:5687–5705
Acknowledgements
Rajashabala Sundaram thanks to the University Grants Commission (UGC) of India for providing financial support to carry out research in the USA under Indo-US Raman Post-Doctoral Fellowship Program and the Department of Science and Technology (India, DST-PURSE Program). HPC center at Utah State University is acknowledged for providing computational facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muniyandi, S., Sundaram, R. & Kar, T. Aluminum doping makes boron nitride nanotubes (BNNTs) an attractive adsorbent of hydrazine (N2H4). Struct Chem 29, 375–382 (2018). https://doi.org/10.1007/s11224-017-1034-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-017-1034-8