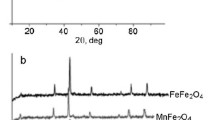
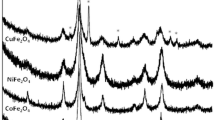
The results of the authors’ investigations of steam reforming of ethanol (SRE) on nanosized ferrites with spinel structure MFe2O4 (M = Mg, Mn, Fe, Co, Ni, Cu, Zn) are summarized. The highest yields of the target product hydrogen were obtained on Mg, Mn, and Fe ferrites. A close to stoichiometric yield of H2 was obtained on nanosized MnFe2O4. A probable scheme for the mechanism of SRE, containing redox and acid–base stages, is proposed.




Similar content being viewed by others
References
J. Sun and Y. Wang, ACS Catal., 4, 1078-1090 (2014).
A. Haryanto, S. Fernando, N. Murali, and S. Adhikari, Energy Fuels, 19, 2098-2106 (2005).
P. D. Vaidya and A. E. Rodrigues, Chem. Eng. J., 117, 39-49 (2006).
M. Ni, D. Y. C. Leung, and M. K. H. Leung, Int. J. Hydrogen Energy, 32, 3238-3247 (2007).
Y. I. Pyatnitsky, L. Yu. Dolgykh, I. L. Stolyarchuk, and P. E. Strizhak, Teor. Éksp. Khim., 49, No. 5, 265-283 (2013). [Theor. Exp. Chem., 49, No. 5, 277-297 (2013) (English translation).]
J. Llorca, P. R. Piscina, J. Sales, et al., Chem. Commun., 641-642 (2001).
M. N. Barroso, M. F. Gomez, L. A. Arrua, et al., Catal. Lett., 109, 13-19 (2006).
H. Muroyama, R. Nakase, T. Matsui, et al., Int. J. Hydrogen Energy, 35, 1575-1581 (2010).
Z. Li, W. Yi, and H. Qun, Trans. Nonferrous Met. Soc. China, 19, 1444-1449 (2009).
S. Q. Chen and Y. Liu, Int. J. Hydrogen Energy, 34, 4735-4746 (2009).
R. Espinal, E. Taboada, E. Molins, et al., Appl. Catal. B, 127, 59-67 (2012).
V. A. de la Pena O’Shea, R. Nafria, P. Ramýrez de la Piscina, et al., Int. J. Hydrogen Energy, 33, 3601-3606 (2008).
I. L. Stolyarchuk, L. Yu. Dolgikh, I. V. Vasilenko, et al. Teor. Éksp. Khim., 48, No. 2, 119-125 (2012). [Theor. Exp. Chem., 48, No. 2, 129-134 (2012) (English translation).]
Y. I. Pyatnitsky, L. Yu. Dolgykh, I. L. Stolyarchuk, and P. E. Strizhak, Teor. Éksp. Khim., 49, No. 2, 99-103 (2013). [Theor. Exp. Chem., 49, No. 2, 109-114 (2013) (English translation).]
L. Yu. Dolgykh, I. L. Stolyarchuk, I. V. Vasylenko, et al. Teor. Éksp. Khim., 49, No. 3, 172-177 (2013). [Theor. Exp. Chem., 49, No. 3, 185-192 (2013) (English translation).]
L. Yu. Dolgykh, I. L. Stolyarchuk, and L. A. Staraya, Teor. Éksp. Khim., 50, No. 4, 244-247 (2014). [Theor. Exp. Chem., 50, No. 4, 245-249 (2014) (English translation).]
I. L. Stolyarchuk, L. Yu. Dolgikh, I. V. Vasilenko, et al., Alternative Sources of Feedstock and Fuel. Collection of Scientific Proceedings of Academy of Sciences of Belarus, Institute of Chemistry of New Materials,V. E. Agabekov, K. N. Gusak, Zh. V. Ignatovich (eds.) [in Russian], Belaruskaya Navuka (2014), No. 1, pp. 186-196.
L. Yu. Dolgykh, I. L. Stolyarchuk, L. A. Staraya, et al., Teor. Éksp. Khim., 51, No. 4, 225-229 (2015). [Theor. Exp. Chem., 51, No. 4, 230-235 (2015) (English translation).]
L. Yu. Dolgykh, I. L. Stolyarchuk, L. A. Staraya, et al., Adsorp. Sci. Technol., 33, Nos. 6-8, 715-721 (2015).
I. L. Stolyarchuk, L. Yu. Dolgykh, I. V. Vasylenko, et al., Teor. Éksp. Khim., 52, No. 4, 244-248 (2016). [Theor. Exp. Chem., 52, No. 4, 246-251 (2016) (English translation).]
L. Yu. Dolgikh, Y. I. Pyatnytsky, and P. E. Strizhak, Bioethanol and Beyond: Advances in Production Process and Future Directions, M. Brienzo (ed.), Nova Sci., New York (2018), Ch. 14, pp. 381-427.
I. V. Vasilenko, K. S. Gavrilenko, I. E. Kotenko, et al., Teor. Éksp. Khim., 43, No. 5, 323-329 (2007). [Theor. Exp. Chem., 43, No. 5, 353-358 (2007) (English translation).]
L. V. Mattos, G. Jacobs, B. H. Davis, and F. B. Noronha, Chem. Rev., 112, 4094-4123 (2012).
P. Ramýrez de la Piscina and N. Homs, Chem. Soc. Rev., 37, 2459-2467 (2008).
C. Trevisanut, M. Mari, J. M. M. Millet, and F. Cavani, J. Hydrogen Energy, 40, 5264-5271 (2015).
C. Trevisanut, F. Bosselet, F. Cavani, and J. M. M. Millet, Catal. Sci. Technol., 5, 1280-1289 (2015).
N. M. Schweitzer, B. Hu, U. Das, et al., ACS Catal., 4, 1091-1092 (2014).
B. Hu, A. Getsoian, N. M. Schweitzer, et al., J. Catal., 322, 24-37 (2015).
B. Hu, N. M. Schweitzer, G. Zhang, et al., ACS Catal., 5, 3494-3503 (2015).
D. J. Elliott and F. Pennella, J. Catal., 119, 359-367 (1989).
T. Nishiguchi, T. Matsumoto, H. Kanai, et al., Appl. Catal. A, 279, 273-277 (2005).
K. Inui, T. Kurabayashi, and S. Sato, J. Catal., 212, 207 (2002).
I. Charkendorff and W. Niemantsverdriet, Concepts of Modern Catalysis and Kinetics, Wiley-VCH, Weinheim (2003).
J. P. Jacobs, A. Maltha, J. G. H. Reitjes, et al., J. Catal., 47, 294-300 (1994).
C. G. Ramankutty and S. Sugunan, Appl. Catal. A, 218, 39-51 (2001).
C. G. Ramankutty, S. Sugunan, B. Thomas, et al., J. Mol. Catal. A, 187, 105-117 (2002).
H. Song, L. Zhang, and U. S. Ozkan, Top. Catal., 55, 1324-1331 (2012).
G. Garbarino, C. Wang, I. Valsamakis, et al., Appl. Catal. B, 174/175, 21-34 (2015).
G. Busca, Chem. Rev., 110, 2217-2249 (2010).
C. L. Kibby and W. K. Hall, J. Catal., 29, 144-159 (1973).
K. Tanabe, M. Misono, Y. Ono, and H. Hattori, New Solid Acids and Bases, Kodansha-Elsevier, Tokyo (1989).
P. Canesson and M. Blanchard, J. Catal., 42, 205-212 (1979).
J. E. Sutton, W. Guo, M. A. Katsoulakis, et al., Nature Chem., 8, 331-337 (2016).
The authors express their gratitude to I. V. Vasylenko for assistance in the synthesis of the catalysts and for investigating the samples by XRD, electron diffraction, and TEM. The work was conducted with financial support from a comprehensive target program of scientific investigations of the National Academy of Sciences of Ukraine “Fundamental aspects of renewable hydrogen energy and fuel cell technologies.”
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Teoreticheskaya i Éksperimental’naya Khimiya, Vol. 54, No. 5, pp. 318-325, September-October, 2018.
Rights and permissions
About this article
Cite this article
Dolgikh, L.Y., Stolyarchuk, I.L., Staraya, L.A. et al. Steam Reforming of Ethanol on Ferrites. Theor Exp Chem 54, 349–357 (2018). https://doi.org/10.1007/s11237-018-9580-8
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11237-018-9580-8